Potassium Dichromate and Chromates play a crucial role in many industrial and laboratory applications due to their powerful oxidizing properties. These chemicals are widely used in industries such as textile manufacturing, leather tanning, water treatment, and chemical synthesis. Despite their usefulness, both Potassium Dichromate and Chromates must be handled with care due to their hazardous nature, particularly their toxicity and carcinogenicity.
Understanding the characteristics and differences between Potassium Dichromate and Chromates is essential for safe handling and effective application. In this article, we will explore what these chemicals are, their common uses, safety considerations, and the distinctions between them.
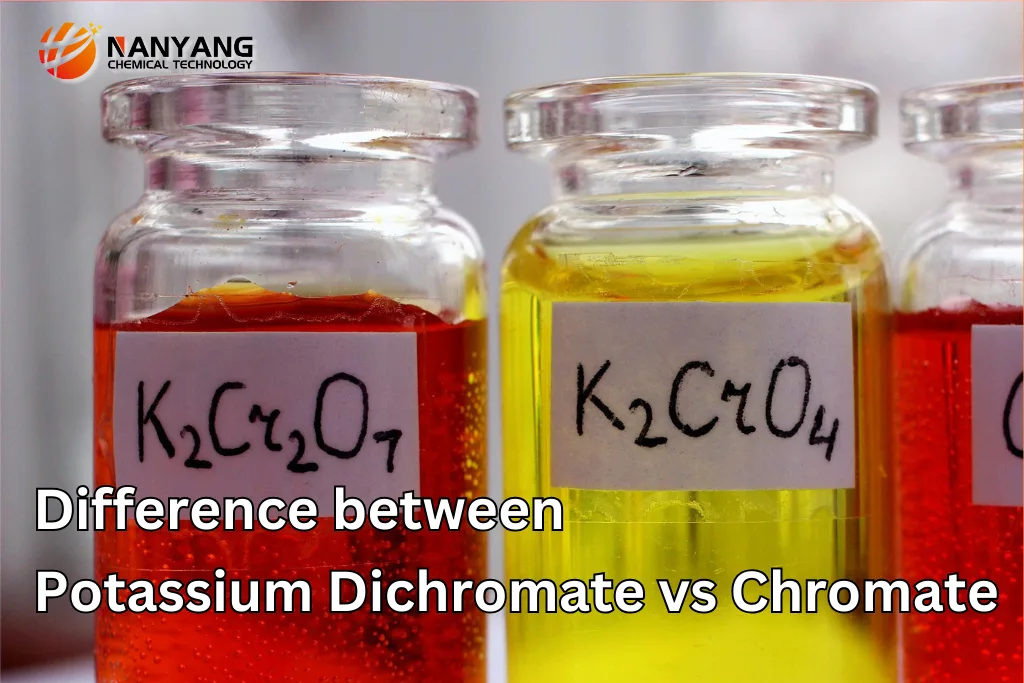
What is Potassium Dichromate?
Potassium Dichromate (K₂Cr₂O₇) is an inorganic chemical compound that appears as orange crystals or a crystalline powder. It is highly soluble in water and is primarily known for its strong oxidizing properties. Potassium Dichromate is used extensively in various industrial and laboratory applications, such as in the production of dyes, pigments, and as an oxidizing agent in chemical reactions.
In laboratories, Potassium Dichromate serves as an important reagent for many chemical syntheses and tests. It is also used in the cleaning and maintenance of laboratory glassware, as its oxidation properties help remove organic contaminants effectively. However, due to its toxic nature, its usage requires strict safety protocols to avoid exposure and environmental harm.
What are Chromates?
Chromates refer to a class of compounds containing chromium in its +6 oxidation state, commonly used in industries such as metal finishing, water treatment, and pigment production. Potassium Chromate (K₂CrO₄) and Sodium Chromate (Na₂CrO₄) are two of the most well-known chromate compounds. These chemicals are generally used in the production of yellow pigments, particularly in the paint and coatings industry.
Chromates also find application in the preservation of metals and alloys, where they serve as corrosion inhibitors. In the field of water treatment, chromates help in removing heavy metals and purifying water supplies. However, like Potassium Dichromate, chromates are also highly toxic and carcinogenic, and their usage is regulated to prevent environmental and health hazards.
Key Applications of Potassium Dichromate and Chromates
Potassium Dichromate is widely used in industries such as leather tanning, where it acts as an oxidizing agent in the process of turning raw hides into durable leather. It is also involved in the textile industry for dyeing and printing fabrics, particularly in the creation of colorfast dyes. In the laboratory, Potassium Dichromate is indispensable for its ability to oxidize various organic compounds, making it a crucial reagent in chemical syntheses.
Chromates, particularly Potassium Chromate, are crucial in the field of chrome plating, where they provide a protective coating on metal surfaces to prevent corrosion and enhance durability. Additionally, chromates are used in the manufacture of pigments such as chrome yellow and chrome green, widely applied in paints, inks, and coatings. They also have an important role in the treatment of industrial wastewater, helping to remove impurities and heavy metals.
Safety Considerations and Handling
Both Potassium Dichromate and Chromates are known for their toxicity and potential carcinogenic effects. Exposure to these chemicals can lead to severe health risks, including respiratory issues, skin burns, and long-term complications such as cancer. It is essential to use appropriate protective equipment, such as gloves, goggles, and respirators, when handling these substances to minimize exposure.
In addition to personal protective measures, safe storage and disposal practices are crucial. Potassium Dichromate and Chromates should be stored in tightly sealed containers, away from incompatible substances such as acids and reducing agents. Disposal must follow strict guidelines to prevent contamination of the environment, especially water sources, where their presence can be detrimental to aquatic life.
Difference Between Potassium Dichromate and Chromates
| Feature | Potassium Dichromate (K₂Cr₂O₇) | Chromates (e.g., Potassium Chromate – K₂CrO₄) |
| Chemical Composition | Contains two chromium atoms in a +6 oxidation state. | Contains chromium in the +6 oxidation state, often as a metal salt. |
| Color | Orange-colored crystals. | Yellow-colored crystals. |
| Common Uses | Used as an oxidizing agent, in textile and leather industries, and as a reagent in laboratories. | Used in chrome plating, water treatment, and pigment production. |
| Toxicity | Highly toxic and carcinogenic, requires careful handling. | Also toxic and carcinogenic, but less commonly used in direct contact applications. |
| Solubility | Soluble in water, highly reactive. | Soluble in water, typically used as a corrosion inhibitor. |
| Stability | Less stable under high pH conditions. | More stable, often used in neutral or alkaline environments. |
| Environmental Impact | Harmful to the environment, especially aquatic life. | Similarly harmful to the environment but used in controlled applications like water treatment. |
Conclusion of Potassium Dichromate and Chromates
Potassium Dichromate and Chromates are versatile and essential chemicals in various industrial applications, but their hazardous nature cannot be overlooked. Proper handling, storage, and disposal are critical to preventing health risks and environmental damage. Understanding their applications, safety concerns, and the differences between these chemicals will ensure their safe and effective use in industry especially when handling reagent product.