In the realm of chemistry, certain compounds stand out for their remarkable ability to interact with metal ions, effectively binding and controlling their behavior. Among these versatile substances, Ethylenediamine Tetraacetic Acid Tetrasodium Salt, commonly known and widely recognized as EDTA-4NA, holds a prominent position. This remarkable chemical, a derivative of the well-known chelating agent Ethylenediamine Tetraacetic Acid (EDTA), plays a crucial role across a diverse spectrum of industrial, laboratory, and even consumer applications, underscoring its significance in modern science and technology.
At its core, EDTA is a polyamino carboxylic acid, a molecule characterized by its capacity to “chelate” – derived from the Greek word for “claw” – metal ions. This chelation process involves the formation of stable, ring-like complexes where the metal ion is held tightly by multiple bonds to the EDTA molecule. The transformation of EDTA into its tetrasodium salt, EDTA-4NA, enhances its water solubility and often makes it more convenient and effective for various applications, particularly in aqueous systems. The four sodium ions neutralize the four carboxyl groups present in the EDTA molecule, resulting in a salt that readily dissolves in water, facilitating its interaction with metal ions in solution.
The exceptional chelating power of Ethylenediamine Tetraacetic Acid Tetrasodium Salt stems from its molecular structure, which features two nitrogen atoms and four carboxylate groups. These six electron-donating atoms can simultaneously coordinate with a single metal ion, forming a highly stable complex. The strength of this interaction, quantified by the stability constant of the complex, varies depending on the specific metal ion involved. This selectivity, though not absolute, allows EDTA-4NA to preferentially bind to certain metal ions over others, making it invaluable in processes ranging from water treatment to analytical chemistry.
The versatility of EDTA-4NA is truly remarkable. In household and industrial cleaning products, it acts as a powerful water softener by sequestering calcium and magnesium ions that contribute to water hardness, preventing the formation of scale and enhancing the effectiveness of detergents and surfactants. Industrially, it finds extensive use in metal finishing processes, preventing corrosion and aiding in electroplating. In the analytical laboratory, Ethylenediamine Tetraacetic Acid Tetrasodium Salt is a fundamental reagent in complexometric titrations, allowing for the precise determination of metal ion concentrations. Even in cosmetic formulations, it plays a role as a stabilizer and preservative by chelating metal ions that could potentially compromise product integrity or color.
This article aims to provide a comprehensive exploration of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA). We will delve into its fundamental chemical properties and structural characteristics, unravel the science behind its potent chelating action, and illuminate its diverse applications across various sectors. Furthermore, we will address crucial aspects related to its safe handling, storage, and the considerations involved in sourcing and purchasing this important chemical compound. By the end of this guide, you will gain a deeper understanding of the multifaceted nature and significance of EDTA-4NA in the modern world.
Chemical Properties and Structure of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA)
To fully appreciate the utility and efficacy of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA), a thorough understanding of its fundamental chemical properties and structural characteristics is essential. This section will delve into the molecular composition, bonding arrangements, and key physical attributes that define this important chemical compound. By examining its chemical formula, structural representation, and various physical parameters, we can gain valuable insights into its behavior and interactions in different chemical environments.
A. Chemical Formula and Molecular Weight:
The anhydrous chemical formula for Ethylenediamine Tetraacetic Acid Tetrasodium Salt is C10H12N2Na4O8. This formula reveals the precise atomic composition of each molecule, indicating the presence of ten carbon atoms, twelve hydrogen atoms, two nitrogen atoms, four sodium atoms, and eight oxygen atoms. It’s crucial to note that EDTA-4NA is often encountered in its hydrated form, meaning that water molecules are incorporated into its crystal lattice. The degree of hydration can vary, and the chemical formula for the hydrated form is typically represented as C10H12N2Na4O8⋅xH2O, where ‘x’ denotes the number of water molecules per molecule of EDTA-4NA. The most common hydrate is the dihydrate (x=2), with the formula C10H12N2Na4O8⋅2H2O. The presence of these water molecules influences the molar mass and certain physical properties of the compound.
The molar mass of anhydrous Ethylenediamine Tetraacetic Acid Tetrasodium Salt (C10H12N2Na4O8) can be calculated by summing the atomic masses of its constituent elements:
(10×12.01)+(12×1.01)+(2×14.01)+(4×22.99)+(8×16.00)=120.1+12.12+28.02+91.96+128.00=380.2g/mol
For the dihydrate form (C10H12N2Na4O8⋅2H2O), the molar mass is:
380.2+(2×18.02)=380.2+36.04=416.24g/mol
Understanding the correct chemical formula and molar mass is essential for accurate stoichiometric calculations in various chemical reactions and analytical procedures involving EDTA-4NA.
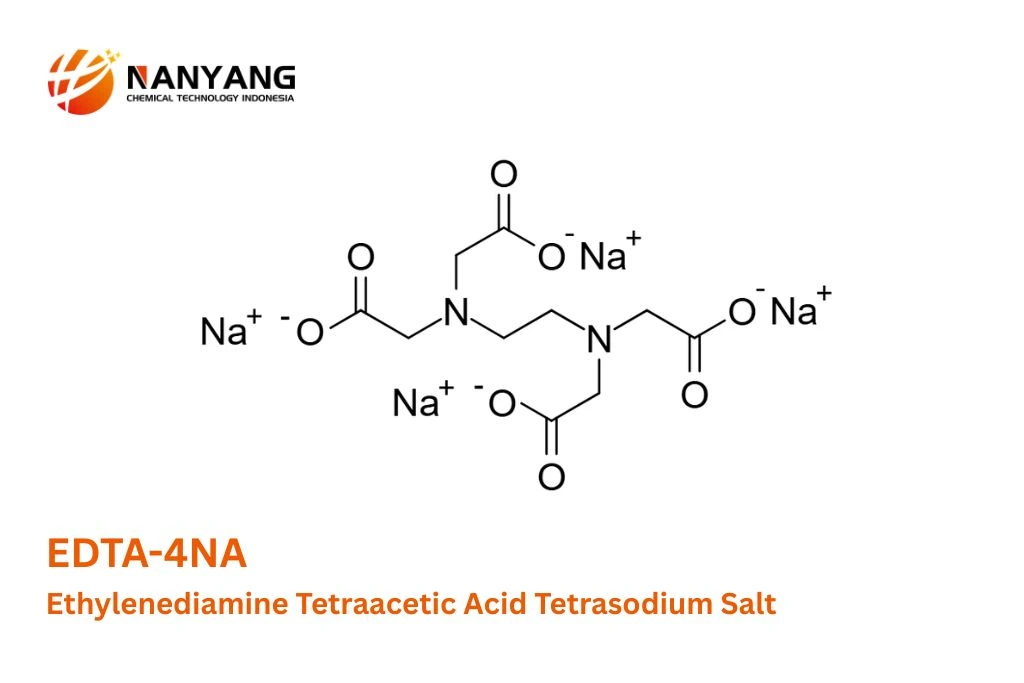
B. Structural Formula and Bonding:
The structural formula of Ethylenediamine Tetraacetic Acid Tetrasodium Salt reveals the spatial arrangement of its atoms and the types of chemical bonds that hold them together. The core of the molecule is derived from ethylenediamine (H2N−CH2−CH2−NH2), where each nitrogen atom is further substituted with two acetic acid groups (−CH2COOH). In EDTA-4NA, the hydrogen atoms of these four carboxylic acid groups are replaced by four sodium cations (Na+).
The structure can be visualized as a central ethylenediamine backbone (N−C−C−N), with each nitrogen atom bonded to two methylene groups (−CH2−). Each of these methylene groups is, in turn, connected to a carboxylate group (−COO−). The negative charge on each carboxylate group is balanced by one of the four positively charged sodium ions. The bonding within the organic part of the molecule involves covalent bonds, where electrons are shared between atoms. The carbon-carbon and carbon-hydrogen bonds are single covalent bonds, while the carbon-oxygen bonds in the carboxylate group exhibit resonance, with partial double bond character between the carbon and both oxygen atoms. The nitrogen atoms form single covalent bonds with the adjacent carbon atoms.
The key feature that makes Ethylenediamine Tetraacetic Acid Tetrasodium Salt such an effective chelating agent is the presence of multiple electron-donating atoms – the two nitrogen atoms and the four oxygen atoms of the carboxylate groups. These atoms possess lone pairs of electrons that can form coordinate covalent bonds with positively charged metal ions. In the chelation process, a single metal ion is simultaneously bound to these multiple donor atoms within the EDTA-4NA molecule, forming a stable, ring-like structure known as a chelate complex. The number of coordination sites available in EDTA-4NA (six in total) allows it to effectively sequester metal ions with various charges and sizes.
The ionic nature of EDTA-4NA is also crucial to its properties. The presence of the four sodium ions contributes to its high water solubility, as the electrostatic interactions between the charged ions and the polar water molecules facilitate dissolution. In aqueous solution, the sodium ions dissociate, leaving behind the negatively charged EDTA anion, which is the active chelating species.
C. Physical Properties:
Ethylenediamine Tetraacetic Acid Tetrasodium Salt typically appears as a white, crystalline powder. It is odorless and has a slightly salty taste. Its most significant physical property is its high solubility in water. The solubility of EDTA-4NA in water is significantly higher than that of the free acid (EDTA), making it more convenient for use in aqueous solutions, which are prevalent in many of its applications. The exact solubility depends on temperature and pH, but it generally exhibits good solubility across a wide range of conditions.
The pH of aqueous solutions of EDTA-4NA is typically alkaline. This is because the tetrasodium salt is the conjugate base of a relatively weak polyprotic acid (EDTA). When dissolved in water, the EDTA anion can undergo hydrolysis, leading to the formation of hydroxide ions (OH−) and thus an increase in pH. The specific pH of a EDTA-4NA solution depends on its concentration.
The stability of Ethylenediamine Tetraacetic Acid Tetrasodium Salt is generally good under normal storage conditions. It is relatively non-volatile and stable at room temperature. However, it should be stored in a cool, dry place to prevent the absorption of moisture, especially for the anhydrous form. Exposure to strong oxidizing agents should also be avoided as it may lead to degradation of the organic component of the molecule.
The particle size and bulk density of EDTA-4NA can vary depending on the manufacturing process. These physical characteristics can be important in certain industrial applications, affecting flowability and handling properties.
In summary, the chemical properties and structure of Ethylenediamine Tetraacetic Acid Tetrasodium Salt are intricately linked to its functionality as a powerful chelating agent. Its defined chemical formula and molar mass allow for precise stoichiometric calculations. Its unique molecular structure, featuring multiple electron-donating atoms, enables the formation of stable complexes with metal ions. Finally, its physical properties, such as high water solubility and alkaline pH in solution, contribute to its versatility and widespread use across numerous fields. Understanding these fundamental aspects provides a solid foundation for exploring the diverse applications of EDTA-4NA.
The Science of Chelation: How Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) Works
The remarkable efficacy of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) in a multitude of applications stems from its fundamental ability to engage in a chemical process known as chelation. This section will delve into the intricate science behind this phenomenon, elucidating how EDTA-4NA interacts with metal ions at a molecular level to form stable complexes, effectively controlling their behavior in various chemical systems. Understanding the principles of chelation is crucial to appreciating the diverse roles played by EDTA-4NA across industries and scientific disciplines.
A. The Concept of Chelation and Coordination Complexes:
Chelation, derived from the Greek word “chele” meaning “claw,” describes the process by which a molecule, known as a chelating agent or chelator, binds to a metal ion through multiple coordination bonds. These bonds are formed between the metal ion, which acts as a Lewis acid (electron pair acceptor), and the electron-donating atoms of the chelating agent, which act as Lewis bases (electron pair donors). The resulting structure, where the metal ion is held in a ring-like fashion by the chelator, is called a chelate complex or a coordination complex.
Ethylenediamine Tetraacetic Acid (EDTA), the parent compound of EDTA-4NA, is a polydentate ligand, meaning it possesses multiple donor atoms capable of coordinating to a single metal ion. Specifically, EDTA has six potential coordination sites: the two nitrogen atoms and the four oxygen atoms of its four carboxylate groups (when deprotonated). This hexadentate nature allows EDTA and its salts, including EDTA-4NA, to form exceptionally stable complexes with many metal ions.
When Ethylenediamine Tetraacetic Acid Tetrasodium Salt dissolves in water, it dissociates into four sodium cations (Na+) and a tetravalent EDTA anion (Y4−), where ‘Y’ represents the fully deprotonated EDTA molecule. It is this negatively charged EDTA anion that is primarily responsible for the chelation process. The sodium ions are spectator ions and do not directly participate in the complex formation with other metal ions.
B. The Binding Mechanism of EDTA-4NA to Metal Ions:
The chelation of metal ions by the EDTA anion involves the formation of coordinate covalent bonds. The lone pairs of electrons on the nitrogen and oxygen atoms of the carboxylate groups are donated to the vacant orbitals of the metal cation, resulting in a stable association. The number of coordinate bonds formed between the EDTA anion and a metal ion can range from four to six, depending on the charge and coordination number of the metal ion.
For instance, when EDTA-4NA encounters a divalent metal ion like calcium (Ca2+) or magnesium (Mg2+), the EDTA anion can wrap around the metal ion, utilizing its six donor atoms to form a stable, cage-like structure. This encapsulation of the metal ion effectively removes it from the solution, preventing it from participating in other chemical reactions or interactions. This is the fundamental principle behind the use of EDTA-4NA as a water softener, where it sequesters calcium and magnesium ions that would otherwise interfere with the action of soaps and detergents and contribute to scale formation.
Similarly, EDTA-4NA can chelate trivalent metal ions like iron (Fe3+) and aluminum (Al3+), forming even more stable complexes due to the higher charge density of these ions and the multiple coordination sites offered by the EDTA anion. This strong affinity for transition metal ions makes EDTA-4NA valuable in applications such as metal cleaning and the prevention of metal-catalyzed degradation reactions.
C. Stability Constants of EDTA Complexes:
The stability of the complexes formed between the EDTA anion and various metal ions is quantified by their formation constants, often expressed as logarithm of the stability constant (logKf). A higher logKf value indicates a greater thermodynamic stability of the complex, meaning the equilibrium of the complex formation reaction lies further to the right, favoring the formation of the chelate.
EDTA exhibits remarkably high stability constants for a wide range of metal ions, particularly for polyvalent cations. The order of stability often follows the Irving-Williams series, which generally correlates with the ionic radius and charge of the metal ion. For example, the logKf values for EDTA complexes with common divalent cations are typically in the range of 8 to 19, indicating very strong binding. The stability constants for trivalent ions are even higher.
The high stability of EDTA metal complexes arises from several factors, including:
- The Chelate Effect: The formation of a chelate complex is thermodynamically more favorable than the binding of monodentate ligands (ligands with only one donor atom) due to an increase in entropy. When a polydentate ligand like EDTA binds to a metal ion, it displaces several water molecules that were previously coordinated to the metal ion. This release of water molecules increases the overall disorder (entropy) of the system, contributing to the negative Gibbs free energy change (ΔG=ΔH−TΔS) and thus a more stable complex.
- Multiple Coordination Sites: The hexadentate nature of the EDTA anion allows for the formation of multiple coordinate bonds with the metal ion, leading to a stronger overall interaction compared to a series of weaker interactions with monodentate ligands.
- Ring Formation: The formation of stable five- or six-membered rings within the chelate complex contributes to its stability. These ring sizes are generally favored due to minimal steric strain and optimal bond angles.
The differing stability constants of EDTA complexes with various metal ions allow for some degree of selectivity in its action. By carefully controlling the pH and the concentration of EDTA, it is sometimes possible to selectively chelate certain metal ions in the presence of others. This selectivity is exploited in analytical techniques like complexometric titrations, where EDTA is used to determine the concentration of specific metal ions in a mixture.
D. Examples of Metal Ion Chelation by EDTA-4NA:
To illustrate the chelation process, consider the interaction of the EDTA anion (Y4−) with a divalent metal ion (M2+):
M2+(aq)+Y4−(aq)⇌[MY]2−(aq)
The equilibrium constant for this reaction is the formation constant (Kf). A high Kf value signifies that the formation of the [MY]2− complex is strongly favored at equilibrium.
Some examples of logKf values for EDTA complexes at 20∘C:
- Ca2+: logKf≈10.7
- Mg2+: logKf≈8.7
- Fe3+: logKf≈25.1
- Cu2+: logKf≈18.8
- Pb2+: logKf≈18.0
These values clearly demonstrate the strong affinity of EDTA for a wide range of metal ions, with particularly high stability for trivalent ions like iron.
In conclusion, the effectiveness of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) as a versatile chemical agent hinges on its ability to participate in chelation. The EDTA anion, derived from EDTA-4NA in aqueous solution, acts as a hexadentate ligand, forming stable, ring-like complexes with metal ions through multiple coordinate bonds. The high stability of these complexes, quantified by their formation constants and influenced by the chelate effect and the polydentate nature of EDTA, allows EDTA-4NA to effectively sequester metal ions, thereby controlling their behavior and enabling its wide-ranging applications in various scientific and industrial processes.
Applications of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) Across Industries
The remarkable chelating properties of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) have made it an indispensable component in a vast array of industrial processes, analytical techniques, and consumer products. Its ability to effectively sequester metal ions allows for the control of their reactivity, solubility, and interaction with other substances, leading to significant benefits in terms of efficiency, quality, and stability. This section will explore the diverse applications of EDTA-4NA across various industries, highlighting its crucial role in addressing specific challenges and enhancing performance.
A. Detergents and Cleaning Products:
One of the most widespread applications of EDTA-4NA is in the formulation of detergents and cleaning products. Hard water, which contains high concentrations of calcium (Ca2+) and magnesium (Mg2+) ions, can significantly reduce the effectiveness of soaps and detergents. These metal ions react with the anionic surfactants in cleaning agents, forming insoluble salts (scum) that precipitate out of solution, reducing the availability of the surfactant to lift dirt and grease.
Ethylenediamine Tetraacetic Acid Tetrasodium Salt acts as a powerful water softener by chelating these calcium and magnesium ions, effectively removing them from the solution and preventing them from interfering with the cleaning process. By sequestering these hardness-causing ions, EDTA-4NA enhances the foaming ability and cleaning power of detergents, allowing them to work more efficiently even in hard water conditions. It also helps to prevent the deposition of mineral scale on surfaces being cleaned, such as laundry, dishes, and bathroom fixtures. Furthermore, EDTA-4NA can chelate other metal ions present in water that might contribute to staining or discoloration. Its inclusion in cleaning formulations contributes to brighter, cleaner results and prevents the build-up of unwanted residues.
B. Industrial Applications:
The industrial sector benefits immensely from the metal-chelating capabilities of Ethylenediamine Tetraacetic Acid Tetrasodium Salt in a variety of processes:
- Metal Cleaning and Electroplating: In metal finishing, EDTA-4NA is used in cleaning solutions to remove metal oxides, scale, and other surface contaminants from metal parts prior to processes like electroplating, painting, or coating. By chelating metal ions, it facilitates the dissolution and removal of these unwanted layers, ensuring a clean and receptive surface for subsequent treatments. In electroplating baths, EDTA-4NA can be used to control the concentration of metal ions and improve the quality and uniformity of the deposited metal layer. It can also prevent the precipitation of metal hydroxides and other insoluble compounds in the plating solution.
- Scale and Corrosion Inhibition: In industrial water systems, such as boilers and cooling towers, the precipitation of mineral salts like calcium carbonate and magnesium silicate can lead to the formation of scale, which reduces heat transfer efficiency and can cause equipment damage. EDTA-4NA is employed as a scale inhibitor by chelating the metal ions responsible for scale formation, preventing their precipitation and deposition on heat exchange surfaces. Similarly, by binding to metal ions on the surface of pipes and equipment, EDTA-4NA can also help to inhibit corrosion, extending the lifespan of industrial infrastructure.
- Pulp and Paper Industry: In the pulp and paper industry, Ethylenediamine Tetraacetic Acid Tetrasodium Salt plays a role in the bleaching process. It helps to stabilize hydrogen peroxide and other bleaching agents by chelating metal ions, such as manganese and iron, which can catalyze their decomposition and reduce bleaching efficiency. By preventing these catalytic reactions, EDTA-4NA ensures a more controlled and effective bleaching process, leading to brighter and higher-quality paper products.
- Textile Processing: In textile processing, EDTA-4NA is used in various stages, including dyeing and finishing. It can help to remove metal ions from the water supply that could interfere with the dyeing process, leading to uneven coloration or dull shades. By chelating these metal ions, EDTA-4NA ensures more consistent and vibrant dyeing results. It can also be used to remove metal contaminants from the fibers themselves, improving the quality of the final textile product.
C. Laboratory and Analytical Chemistry:
Ethylenediamine Tetraacetic Acid Tetrasodium Salt is a fundamental reagent in laboratory and analytical chemistry due to its predictable stoichiometry in metal complex formation and the high stability of the resulting chelates:
- Complexometric Titrations: EDTA, and by extension EDTA-4NA after dissolution, is the basis of complexometric titrations, a widely used analytical technique for determining the concentration of metal ions in solution. The EDTA anion reacts with metal ions in a 1:1 stoichiometric ratio (in most cases), allowing for precise quantification based on the volume of EDTA solution required to reach the endpoint of the titration. Various indicators are used to detect the endpoint, which corresponds to the complete chelation of the metal ion being analyzed. This method is employed for determining the hardness of water, the concentration of metal ions in environmental samples, and in various quality control applications.
- Preparation of Buffer Solutions: While not a direct chelating application, the sodium ions of EDTA-4NA can contribute to the ionic strength of buffer solutions used in various biochemical and chemical experiments. The EDTA itself might be included in some buffer formulations to chelate trace metal ions that could interfere with enzymatic reactions or other sensitive processes.
- Metal Ion Masking: In analytical procedures where the presence of certain metal ions might interfere with the determination of others, EDTA-4NA can be used as a masking agent. By selectively chelating the interfering metal ions, it prevents them from reacting with the reagents used in the analysis of the target ion, ensuring accurate results.
D. Cosmetics and Personal Care Products:
In the cosmetics and personal care industry, Ethylenediamine Tetraacetic Acid Tetrasodium Salt is used primarily as a chelating agent to enhance product stability and prevent unwanted interactions:
- Preservative and Stabilizer: Metal ions present as trace contaminants in raw materials or introduced during the manufacturing process can catalyze the degradation of cosmetic ingredients, leading to changes in color, odor, texture, and efficacy. EDTA-4NA chelates these metal ions, effectively deactivating them and preventing them from promoting these degradation reactions, thereby extending the shelf life and maintaining the quality of the cosmetic product.
- Enhancing Foaming and Cleansing: Similar to its role in detergents, EDTA-4NA can improve the foaming and cleansing properties of shampoos, soaps, and other personal care products by softening the water and preventing the formation of insoluble salts with surfactants.
E. Food Industry (Limited Use):
While its use is regulated and limited, Ethylenediamine Tetraacetic Acid Tetrasodium Salt has some applications in the food industry as a preservative and color retention agent. It can chelate metal ions that might catalyze oxidation reactions leading to food spoilage or discoloration. However, due to safety considerations and regulatory restrictions, its use in food products is carefully controlled and typically limited to specific applications and concentrations.
F. Agriculture (Micronutrient Delivery):
In agriculture, EDTA-4NA, and other EDTA salts, are used to chelate essential micronutrients like iron, zinc, manganese, and copper in fertilizers. Chelating these metal ions helps to keep them soluble in the soil and prevents them from being converted into insoluble forms that are unavailable to plants. This enhanced solubility and bioavailability ensure that plants can effectively absorb these vital nutrients, promoting healthy growth and increasing crop yields.
In conclusion, the versatility of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) stems directly from its potent chelating ability. Its applications span a wide range of industries, from household cleaning to sophisticated analytical techniques and industrial processes. By effectively controlling the behavior of metal ions, EDTA-4NA plays a crucial role in enhancing the performance, stability, and quality of countless products and processes that underpin modern society. Its continued importance is a testament to the fundamental principles of coordination chemistry and the practical benefits of metal ion sequestration.
Safety, Handling, and Storage of Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA)
While Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) is a widely used and generally considered safe chemical when handled appropriately, it is crucial to understand and adhere to proper safety protocols, handling procedures, and storage guidelines to minimize potential risks to human health and the environment. This section will detail the potential hazards associated with EDTA-4NA, outline recommended handling practices, and provide guidance on appropriate storage conditions to ensure its safe and effective use.
A. Potential Hazards and Safety Precautions:
Exposure to Ethylenediamine Tetraacetic Acid Tetrasodium Salt can pose certain health hazards, primarily related to its irritant properties. The severity of these effects can vary depending on the route and duration of exposure, as well as the concentration of the substance.
- Skin Contact: Direct contact with EDTA-4NA in its solid form or concentrated solutions can cause skin irritation. This may manifest as redness, itching, and mild inflammation. Prolonged or repeated exposure may lead to more severe irritation or even dermatitis in sensitive individuals. It is therefore essential to avoid direct skin contact and wear appropriate personal protective equipment (PPE) when handling this chemical.
- Eye Contact: Eye contact with EDTA-4NA can cause significant irritation, characterized by redness, watering, pain, and potentially corneal abrasion if the solid material is rubbed into the eye. Immediate and thorough flushing of the eyes with copious amounts of water is crucial in case of accidental exposure. Medical attention should be sought promptly.
- Inhalation: Inhalation of EDTA-4NA dust or fine particles can irritate the respiratory tract, causing coughing, sneezing, and shortness of breath. Individuals with pre-existing respiratory conditions may be more susceptible to these effects. It is important to handle EDTA-4NA in well-ventilated areas or use respiratory protection, such as a dust mask or respirator, when handling the powdered form, especially during weighing or transfer operations.
- Ingestion: Ingestion of small amounts of EDTA-4NA may cause mild gastrointestinal irritation, including nausea and vomiting. However, ingestion of larger quantities could lead to more significant systemic effects due to the chelating properties of EDTA. While its acute toxicity is generally considered low, the potential for it to bind essential metal ions in the body warrants caution. In case of accidental ingestion, medical advice should be sought.
- Environmental Hazards: While EDTA-4NA is biodegradable under certain conditions, its persistence in the environment and its ability to chelate heavy metals raise some environmental concerns. The chelation of heavy metals in soil and water could potentially increase their mobility and bioavailability, leading to contamination of ecosystems. Therefore, it is important to prevent the release of significant quantities of EDTA-4NA into the environment and to dispose of waste containing this chemical in accordance with local regulations.
To mitigate these potential hazards, several safety precautions should be implemented when handling Ethylenediamine Tetraacetic Acid Tetrasodium Salt:
- Personal Protective Equipment (PPE): Always wear appropriate PPE, including safety glasses with side shields, chemical-resistant gloves (e.g., nitrile or neoprene), and a lab coat or apron to prevent skin and eye contact. If handling the powdered form, a dust mask or respirator may also be necessary to avoid inhalation.
- Ventilation: Ensure adequate ventilation when working with EDTA-4NA, especially when handling the powdered form or preparing solutions. If necessary, use a fume hood or local exhaust ventilation to minimize the concentration of airborne particles.
- Hygiene Practices: Practice good personal hygiene. Avoid eating, drinking, or smoking in areas where EDTA-4NA is handled. Wash hands thoroughly with soap and water after handling the chemical and before eating, drinking, or using the restroom.
- Spill Response: In case of a spill, contain the material and clean it up promptly using appropriate methods. Avoid generating dust if the spill involves the solid form. Sweep or vacuum the material carefully and place it in a designated waste container. Rinse the spill area with water. For large spills, consult the Safety Data Sheet (SDS) for specific cleanup procedures.
- First Aid Measures: Ensure that safety showers and eyewash stations are readily accessible in areas where EDTA-4NA is handled. Know the appropriate first aid procedures for skin contact, eye contact, inhalation, and ingestion, and have the SDS readily available for reference.
B. Proper Handling Procedures:
Safe handling of Ethylenediamine Tetraacetic Acid Tetrasodium Salt involves adopting practices that minimize the risk of exposure and prevent accidents:
- Read the Safety Data Sheet (SDS): Before handling EDTA-4NA, thoroughly review the SDS provided by the manufacturer. The SDS contains detailed information on the chemical’s hazards, safe handling procedures, storage requirements, and emergency response measures.
- Use Proper Containers and Equipment: Ensure that containers used for storing and handling EDTA-4NA are properly labeled, compatible with the chemical, and in good condition. Use appropriate weighing and measuring equipment to avoid spills and minimize dust generation.
- Avoid Dust Formation: When handling the powdered form, take precautions to minimize the generation of dust. Work slowly and carefully, and consider using techniques like wet wiping or vacuuming with a HEPA filter for cleanup.
- Prepare Solutions Carefully: When preparing solutions of EDTA-4NA, add the solid slowly to the solvent with stirring to avoid splashing and ensure complete dissolution. Use appropriate personal protective equipment during this process.
- Avoid Mixing with Incompatible Materials: Consult the SDS for information on incompatible materials. EDTA-4NA may react with strong oxidizing agents and strong acids. Avoid mixing it with such substances.
- Minimize Contact: Handle EDTA-4NA in a manner that minimizes direct contact with skin, eyes, and clothing. Use tools such as spatulas or scoops for transferring the solid material.
- Labeling: Ensure that all containers of EDTA-4NA, including stock solutions and working solutions, are clearly and accurately labeled with the chemical name, concentration (if applicable), hazard warnings, and date of preparation.
C. Recommended Storage Conditions:
Proper storage of Ethylenediamine Tetraacetic Acid Tetrasodium Salt is essential to maintain its stability, prevent degradation, and minimize potential hazards:
- Storage Area: Store EDTA-4NA in a cool, dry, and well-ventilated area, away from direct sunlight, heat sources, and incompatible materials. The storage area should be protected from moisture to prevent caking or degradation of the compound, especially for the anhydrous form.
- Container Integrity: Store EDTA-4NA in tightly closed, properly labeled containers made of compatible materials. Avoid storing it in damaged or improperly sealed containers.
- Segregation: Keep EDTA-4NA segregated from incompatible substances, such as strong oxidizing agents and strong acids, as indicated in the SDS. Store it away from food and beverages.
- Temperature and Humidity: While EDTA-4NA is generally stable at room temperature, maintaining a consistent and moderate temperature and low humidity in the storage area will help to prolong its shelf life and prevent degradation.
- Spill Containment: The storage area should be designed to contain any potential spills or leaks. This may involve the use of spill trays or other containment measures.
- Regular Inspections: Regularly inspect storage containers for any signs of damage or leakage. Promptly address any issues to prevent accidents or environmental contamination.
- Shelf Life: While EDTA-4NA is generally stable, it is good practice to follow the manufacturer’s recommendations regarding shelf life and to use older stock before newer stock.
By adhering to these safety, handling, and storage guidelines, users can significantly minimize the risks associated with Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) and ensure its safe and effective use in a wide range of applications. Always prioritize safety and consult the Safety Data Sheet for the most comprehensive and up-to-date information.
Sourcing and Purchasing Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA)
For businesses, researchers, and other entities requiring Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA), the process of sourcing and purchasing this chemical involves several key considerations to ensure they obtain the right quality, quantity, and grade from a reliable supplier at a competitive price. This section will guide potential buyers through the essential steps and factors to consider when sourcing and purchasing EDTA-4NA.
A. Identifying Potential Suppliers:
The first step in the purchasing process is to identify potential suppliers of Ethylenediamine Tetraacetic Acid Tetrasodium Salt. Several types of vendors typically offer this chemical:
- Manufacturers: These companies produce EDTA-4NA directly. Purchasing from a manufacturer can often offer the most competitive pricing, especially for large quantities. Manufacturers can also provide detailed technical specifications and may be able to accommodate specific purity or grade requirements. However, they may have minimum order quantities that are not suitable for smaller users. Identifying manufacturers can be done through online searches using keywords like “Ethylenediamine Tetraacetic Acid Tetrasodium Salt manufacturer” or by consulting chemical industry directories and trade publications.
- Distributors and Wholesalers: Chemical distributors and wholesalers purchase EDTA-4NA in bulk from manufacturers and resell it to end-users in smaller quantities. They often offer a wider range of package sizes and may provide additional services such as technical support and local warehousing, which can lead to faster delivery times. Distributors can be a good option for businesses that require moderate quantities or prefer the convenience of dealing with a local supplier. Online searches using terms like “EDTA-4NA supplier” or “EDTA Tetrasodium Salt distributor” can help identify these vendors.
- Online Chemical Suppliers: The rise of e-commerce has led to the emergence of numerous online chemical suppliers that offer a wide variety of chemicals, including EDTA-4NA. These platforms can provide a convenient way to compare prices and product specifications from multiple vendors. However, it is crucial to vet online suppliers carefully to ensure their reliability, the quality of their products, and their adherence to safety and regulatory standards. Look for established online suppliers with transparent product information, secure payment gateways, and clear shipping and return policies.
- Specialty Chemical Suppliers: Some suppliers specialize in specific types of chemicals or cater to particular industries. If your application requires a specific grade or formulation of EDTA-4NA, it may be beneficial to seek out specialty chemical suppliers who have expertise in that area.
B. Factors to Consider When Choosing a Supplier:
Once potential suppliers have been identified, several factors should be carefully evaluated before making a purchasing decision:
- Purity and Grade of the Product: Ethylenediamine Tetraacetic Acid Tetrasodium Salt is available in various purity grades, such as technical grade, reagent grade, and ACS (American Chemical Society) grade. The required purity will depend on the specific application. For analytical work or applications where impurities could interfere with the process, a higher purity grade is essential. Always verify the product specifications provided by the supplier and request a Certificate of Analysis (CoA) to confirm the actual purity and quality of the batch.
- Pricing and Bulk Discounts: Obtain quotes from multiple suppliers to compare pricing. Consider the total cost, including the price per unit, shipping and handling charges, and any applicable taxes or fees. Inquire about potential discounts for bulk purchases, as buying larger quantities can often lead to significant cost savings over time. However, ensure that your storage capacity and usage rate justify a bulk purchase to avoid product degradation over an extended period.
- Shipping and Handling Costs and Logistics: Shipping costs for chemicals can vary significantly depending on the weight, volume, and destination. Inquire about the supplier’s shipping options, estimated delivery times, and handling procedures, especially if you have specific requirements or deadlines. Consider the supplier’s location relative to your facility, as closer suppliers may offer faster and more cost-effective shipping.
- Supplier Reputation and Certifications: Research the reputation and reliability of potential suppliers. Look for customer reviews, testimonials, and industry ratings. Check if the supplier holds relevant certifications, such as ISO certifications, which can indicate their commitment to quality management and environmental standards. A well-established supplier with a positive track record is more likely to provide consistent product quality and reliable service.
- Availability and Lead Times: Confirm the supplier’s stock availability and lead times for delivery. If your application requires a continuous supply of EDTA-4NA, ensure that the supplier has a stable supply chain and can meet your ongoing needs. Inquire about their order processing times and typical delivery schedules.
- Technical Support and Documentation: Depending on your application, the availability of technical support and comprehensive documentation, such as Material Safety Data Sheets (MSDS/SDS) and Certificates of Analysis (CoA), can be crucial. A knowledgeable supplier can provide valuable assistance with product selection, handling guidelines, and troubleshooting.
- Payment Terms and Conditions: Understand the supplier’s payment terms, including accepted payment methods, credit options, and payment schedules. Ensure that the terms are favorable and align with your company’s financial policies.
C. Understanding Product Specifications and Certificates of Analysis (CoA):
Before finalizing a purchase, it is essential to thoroughly review the product specifications and request a Certificate of Analysis (CoA) from the supplier.
- Product Specifications: These documents outline the key characteristics of the EDTA-4NA being offered, including its purity, assay (percentage of active ingredient), moisture content, pH, heavy metal content, and other relevant parameters. Ensure that the specifications meet the requirements of your intended application. Pay close attention to the grade of the product and any specific limits on impurities.
- Certificate of Analysis (CoA): The CoA is a batch-specific document provided by the manufacturer or supplier that details the actual results of quality control testing performed on a particular batch of EDTA-4NA. It confirms that the product meets the specified purity and quality standards. The CoA typically includes the batch number, date of analysis, test methods used, and the measured values for each specified parameter. Always request and carefully review the CoA for the specific batch you are purchasing to ensure it meets your requirements.
D. Purchase Order and Quality Control:
Once a supplier has been selected and the product specifications and pricing have been agreed upon, a formal purchase order should be issued. The purchase order should clearly specify the product name (Ethylenediamine Tetraacetic Acid Tetrasodium Salt or EDTA-4NA), the desired quantity, the required grade and purity, the agreed-upon price, shipping instructions, and any other relevant terms and conditions.
Upon receiving the shipment, it is advisable to perform your own quality control checks to ensure that the delivered product meets the agreed-upon specifications and matches the information provided in the CoA. This may involve visual inspection, pH testing of a solution, or more sophisticated analytical tests if your application requires stringent quality control. Any discrepancies should be promptly reported to the supplier.
By carefully considering these factors and following a systematic approach to sourcing and purchasing Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA), businesses and researchers can ensure they obtain a high-quality product from a reliable supplier at a competitive price, meeting their specific application needs while adhering to safety and regulatory requirements. Building a strong and transparent relationship with your supplier is also crucial for ensuring a consistent and reliable supply of this important chemical.
Conclusion
In conclusion, Ethylenediamine Tetraacetic Acid Tetrasodium Salt (EDTA-4NA) stands as a remarkably versatile and indispensable chemical compound with a broad spectrum of applications across numerous industries and scientific disciplines. Its exceptional ability to chelate metal ions, a direct consequence of its unique molecular structure and the principles of coordination chemistry, underpins its efficacy in diverse processes ranging from water softening and industrial cleaning to analytical chemistry and even the stabilization of cosmetic formulations. The introduction of sodium ions to the EDTA molecule significantly enhances its water solubility, making EDTA-4NA particularly well-suited for aqueous systems, which are prevalent in many of its key applications.
The detailed exploration of its chemical properties and structure reveals the fundamental basis for its chelating action, while the examination of its applications highlights its crucial role in enhancing efficiency, preventing unwanted reactions, and ensuring the quality of various products and processes. From household detergents that perform optimally in hard water to sophisticated analytical techniques that rely on its predictable metal-binding behavior, EDTA-4NA has become an integral component of modern technology and everyday life.
However, as with any chemical substance, responsible handling, storage, and awareness of potential hazards are paramount. Adhering to established safety protocols and consulting the Safety Data Sheet are essential for minimizing risks to human health and the environment. Furthermore, the sourcing and purchasing of Ethylenediamine Tetraacetic Acid Tetrasodium Salt require careful consideration of factors such as purity, grade, supplier reliability, and cost-effectiveness to ensure that the chosen product meets the specific needs of the intended application.
The continued relevance and widespread use of EDTA-4NA underscore the importance of understanding its fundamental properties and its multifaceted interactions with metal ions. As industries continue to evolve and new challenges emerge, the unique chelating capabilities of Ethylenediamine Tetraacetic Acid Tetrasodium Salt will likely continue to be leveraged for innovative solutions, solidifying its position as a cornerstone of applied chemistry for years to come.