From the zesty tang that awakens your taste buds in a freshly squeezed lemonade to its indispensable role in maintaining the pristine shine of your household appliances, citric acid is a ubiquitous compound that silently enhances our daily lives. While many recognize its natural abundance in citrus fruits like lemons and limes, the commercially available form often takes the crystalline structure of monohydrate citric acid. This specific form, where each molecule of citric acid elegantly binds with one molecule of water within its crystal lattice, possesses a unique set of properties that make it a cornerstone ingredient across a surprisingly diverse spectrum of industries. Understanding the nuances of monohydrate citric acid, its chemical composition, and its distinct characteristics is key to appreciating its widespread utility and the vital functions it performs.
Delving deeper than its simple association with sourness, monohydrate citric acid, with its chemical formula C6H8O7⋅H2O, is a weak organic tricarboxylic acid. The presence of that single water molecule, the “monohydrate” aspect, distinguishes it from its anhydrous counterpart and influences certain physical properties, such as its crystal structure and stability under specific conditions. This seemingly minor difference can have practical implications for its handling, storage, and suitability for particular applications. As we navigate the intricate world of chemical compounds, recognizing these subtle yet significant distinctions becomes paramount.
The journey of monohydrate citric acid from the juice of citrus fruits to the vast array of products we encounter daily is a testament to its versatility and effectiveness. In the realm of food and beverage, it acts as a natural preservative, a flavor enhancer providing that characteristic tartness, and an antioxidant, contributing to the stability and appeal of countless products. From carbonated drinks and confectioneries to processed foods and even wine production, monohydrate citric acid plays an indispensable role in ensuring both taste and longevity.
Beyond the culinary landscape, the applications of monohydrate citric acid extend into the domain of cleaning and industrial processes. Its inherent acidity and ability to chelate metal ions make it a powerful yet environmentally friendlier alternative to harsher chemicals in descaling solutions, rust removal, and general cleaning agents. This biodegradable nature aligns with the growing global emphasis on sustainable practices, further solidifying its importance in modern industrial applications. Moreover, the pharmaceutical and nutraceutical industries leverage the unique properties of monohydrate citric acid in the formulation of effervescent tablets, as an anticoagulant in blood storage, and as a pH adjuster in various medications, highlighting its crucial role in healthcare.
This article aims to unravel the multifaceted nature of monohydrate citric acid, moving beyond its basic definition to explore its fundamental chemical properties, its diverse applications across various sectors, and the essential considerations for its safe handling and storage. By providing a comprehensive overview, we seek to illuminate the power and versatility of this seemingly simple compound that silently contributes to the quality, safety, and enjoyment of countless aspects of our modern world. Join us as we delve into the science and applications that make monohydrate citric acid a truly indispensable chemical substance.
- Understanding Monohydrate Citric Acid
- Diverse Applications of Monohydrate Citric Acid
- Safety, Handling, and Storage of Monohydrate Citric Acid
- Sourcing and Purchasing Monohydrate Citric Acid
- How is Monohydrate Citric Acid Produced?
- Applications of Monohydrate Citric Acid in Various Industries
- Safety and Regulatory Approval
- Differences Between Monohydrate Citric Acid and Anhydrous Citric Acid
- Proper Storage and Handling
- FAQ (Frequently Asked Questions)
- Conclusion
Understanding Monohydrate Citric Acid
To truly appreciate the versatility of monohydrate citric acid, it is essential to delve into its fundamental chemical structure and the resulting physical and chemical properties that dictate its behavior and applications. This section will provide a comprehensive exploration of what monohydrate citric acid is at a molecular level, how its structure influences its characteristics, and how it compares to its closely related anhydrous form.
A. Chemical Structure and Formula: The Blueprint of Monohydrate Citric Acid
At its core, citric acid is a tricarboxylic acid, meaning it possesses three carboxyl (-COOH) functional groups. These groups are responsible for its acidic nature and its ability to participate in a variety of chemical reactions. The anhydrous form of citric acid has the molecular formula C6H8O7, indicating six carbon atoms, eight hydrogen atoms, and seven oxygen atoms arranged in a specific molecular structure.
However, monohydrate citric acid, the focus of our discussion, incorporates an additional element into its crystalline structure: a single molecule of water (H2O) for every molecule of citric acid. This water molecule is not chemically bonded to the citric acid molecule in the traditional sense but is rather trapped within the crystal lattice through intermolecular forces, primarily hydrogen bonds. This inclusion of water is explicitly denoted in the chemical formula as C6H8O7⋅H2O. The dot in the formula signifies that the water molecule is associated with the citric acid molecule in a specific stoichiometric ratio within the crystal structure.
Understanding this structural detail is crucial because the presence of this water of hydration influences several physical properties of monohydrate citric acid, such as its crystal shape, melting point, and stability. The water molecules contribute to the overall arrangement of the citric acid molecules in the solid state, forming a distinct crystalline structure. This ordered arrangement dictates how the substance interacts with other molecules and its macroscopic properties.
B. Physical and Chemical Properties: Manifestations of Molecular Structure
The unique molecular architecture of monohydrate citric acid gives rise to a distinct set of physical and chemical properties that are critical to its diverse applications:
- Appearance: At room temperature, monohydrate citric acid typically presents as colorless or white, translucent crystals or a crystalline powder. The crystal shape can vary depending on the crystallization conditions, often appearing as rhombic dodecahedra or prisms. This crystalline form is a direct consequence of the ordered arrangement of citric acid and water molecules within the crystal lattice.
- Solubility: Monohydrate citric acid exhibits high solubility in water due to its polar nature and its ability to form hydrogen bonds with water molecules. This high water solubility is a key factor in its effectiveness in aqueous solutions, such as in beverages, cleaning products, and certain industrial processes. Its solubility in organic solvents is generally lower and varies depending on the polarity of the solvent.
- Acidity (pH Level): As a tricarboxylic acid, monohydrate citric acid is a weak acid, meaning it does not completely dissociate into its ions when dissolved in water. It has three dissociable protons, each with its own acid dissociation constant (Ka). These Ka values determine the pH of its solutions and its buffering capacity. The acidity of monohydrate citric acid is fundamental to its role as a flavor enhancer, preservative, and pH adjuster in various applications. The typical pH of a 1% aqueous solution of citric acid is around 2.2.
- Hygroscopicity: Monohydrate citric acid is mildly hygroscopic, meaning it can absorb moisture from the surrounding air. This property needs to be considered during storage to prevent caking and maintain its purity and free-flowing characteristics. Proper packaging and storage in low-humidity environments are essential to mitigate this effect.
- Melting Point: The presence of the water of hydration in monohydrate citric acid significantly affects its melting point compared to anhydrous citric acid. Monohydrate citric acid typically melts around 100∘C (212∘F), at which point it loses its water of crystallization and then decomposes at higher temperatures. Anhydrous citric acid, lacking the water molecules within its crystal structure, has a higher melting point of approximately 153∘C (307∘F). This difference in melting point can be a crucial factor in selecting the appropriate form for specific applications or processing conditions.
- Chemical Reactivity: The carboxyl groups in monohydrate citric acid allow it to participate in a variety of chemical reactions, including esterification (reaction with alcohols to form esters), salt formation (reaction with bases to form citrates), and decarboxylation (loss of carbon dioxide upon heating). These reactions are fundamental to its use in various industrial processes and in the formation of citrate salts, which have their own distinct applications. Its ability to chelate metal ions, forming stable complexes, is particularly important in applications such as water treatment and as an antioxidant synergist.
C. Monohydrate vs. Anhydrous Citric Acid: Understanding the Key Distinction
The distinction between monohydrate citric acid and anhydrous citric acid lies solely in the presence or absence of the water molecule within the crystal lattice. While both forms consist of the same citric acid molecule, this structural difference leads to variations in their physical properties, which, in turn, influence their suitability for different applications.
- Water Content and Stability: Monohydrate citric acid contains approximately 7.6% water by weight due to the water of hydration. When heated above its melting point, this water is released, and the compound essentially becomes anhydrous before further decomposition occurs. Anhydrous citric acid, by definition, contains no water of crystallization and is generally more stable at higher temperatures before melting.
- Handling and Flowability: The crystalline nature of monohydrate citric acid can sometimes lead to caking due to its mild hygroscopicity. Anhydrous citric acid, being less prone to absorbing moisture, often exhibits better flowability and is preferred in applications where consistent powder handling is crucial.
- Solubility Rate: While both forms are highly soluble in water, the rate of dissolution might differ slightly due to the initial energy required to break down the crystal lattice, which includes the water molecules in the monohydrate form.
- Applications: While both forms share many applications, the specific form chosen often depends on the desired physical properties or the manufacturing process. For instance, anhydrous citric acid might be preferred in applications where a lower water content is desired or where processing involves higher temperatures. Monohydrate citric acid, being the more commonly produced and widely available form, is suitable for a vast majority of applications, particularly those involving aqueous solutions or where the water of hydration does not pose a significant issue.
In conclusion, understanding the chemical structure and the resulting physical and chemical properties of monohydrate citric acid, along with its key differences from anhydrous citric acid, provides a crucial foundation for appreciating its diverse applications and for making informed decisions regarding its use in various industrial, food, and pharmaceutical processes. The presence of that single water molecule within its crystalline structure, while seemingly minor, imparts unique characteristics that contribute significantly to the multifaceted world of this essential chemical compound.
Diverse Applications of Monohydrate Citric Acid
The unique properties of monohydrate citric acid, stemming from its molecular structure and resulting chemical behavior, have paved the way for its widespread adoption across a remarkable array of industries. From the everyday foods we consume to the sophisticated processes in pharmaceutical manufacturing and the cleaning agents that maintain hygiene, monohydrate citric acid plays a crucial, often незаметную, role. This section will delve into the diverse applications of this versatile compound, highlighting its functional significance in various sectors.
A. Food and Beverage Industry: Enhancing Flavor, Preservation, and Quality
The food and beverage industry stands as one of the largest consumers of monohydrate citric acid, leveraging its natural acidity and other beneficial properties to enhance the taste, safety, and shelf life of countless products.
- Acidulant: Perhaps the most recognizable role of monohydrate citric acid in this sector is as an acidulant. It imparts a clean, tart, and refreshing sour taste that is highly desirable in a wide variety of beverages, including carbonated soft drinks, fruit juices, iced teas, and sports drinks. It balances sweetness, enhances other flavor notes, and contributes to the overall palatability of these products. In confectionery, monohydrate citric acid provides the characteristic sourness in candies, gummies, and sour powders, creating a sensory experience that consumers enjoy. It is also used in jams, jellies, and preserves to lower the pH, which aids in pectin gel formation and contributes to their characteristic tang.
- Preservative: The acidic environment created by the addition of monohydrate citric acid inhibits the growth of many spoilage-causing bacteria, yeasts, and molds. By lowering the pH below the optimal range for these microorganisms, it effectively acts as a natural preservative, extending the shelf life of various food and beverage products. This is particularly important in processed foods, canned goods, and fermented products, contributing to food safety and reducing waste.
- Antioxidant Synergist: While not a primary antioxidant itself, monohydrate citric acid can enhance the effectiveness of other antioxidants, such as ascorbic acid (vitamin C). It acts as a chelating agent, binding to trace metal ions that can catalyze oxidation reactions, thereby preventing the degradation of fats, oils, and other sensitive food components. This synergistic effect helps to maintain the color, flavor, and nutritional value of food products over time.
- Emulsifier and Stabilizer: In certain food formulations, monohydrate citric acid and its salts (citrates) can act as emulsifiers and stabilizers. They can help to maintain the dispersion of oil and water phases in emulsions like sauces and dressings, preventing separation and ensuring a consistent texture. They can also interact with proteins and starches, contributing to the stability and texture of processed foods.
- Other Applications: Monohydrate citric acid finds use in various other niche applications within the food industry, such as in the production of cheese to adjust acidity during the cheesemaking process and as a processing aid in the cleaning of food processing equipment.
B. Cleaning Products: A Biodegradable and Effective Cleaning Agent
Beyond the realm of food, monohydrate citric acid has emerged as a valuable and environmentally friendly ingredient in a wide range of cleaning products for both household and industrial use.
- Descaler: One of the most prominent applications in cleaning is its effectiveness as a descaling agent. Monohydrate citric acid readily reacts with mineral deposits, such as calcium and magnesium carbonates (limescale), that accumulate in kettles, coffee makers, dishwashers, washing machines, and bathroom fixtures. The acidic nature of monohydrate citric acid dissolves these mineral scales, restoring the efficiency and performance of the appliances. Its biodegradability makes it a preferred alternative to harsher, less environmentally friendly descaling agents.
- Rust Remover: Monohydrate citric acid can also be used to remove light rust stains from various surfaces. It reacts with the iron oxides that constitute rust, dissolving them and leaving the underlying material clean. While it may not be as aggressive as strong mineral acids, its gentler action makes it suitable for more delicate surfaces.
- pH Adjuster: In many cleaning formulations, monohydrate citric acid is used to adjust the pH of the product. Maintaining the optimal pH is crucial for the effectiveness of other cleaning ingredients, such as surfactants and enzymes. By buffering the solution, monohydrate citric acid helps to ensure consistent cleaning performance.
- General Cleaner: Due to its mild acidity and chelating properties, monohydrate citric acid can be incorporated into all-purpose cleaners for removing soap scum, hard water stains, and other household grime. Its relatively low toxicity and natural origin make it a desirable ingredient in eco-friendly cleaning products.
C. Pharmaceutical and Nutraceutical Industry: Essential for Formulation and Functionality
The pharmaceutical and nutraceutical industries rely on the unique properties of monohydrate citric acid for various crucial applications.
- Effervescent Tablets and Powders: Monohydrate citric acid is a key component in effervescent formulations. When combined with bicarbonates (such as sodium bicarbonate or potassium bicarbonate) in the presence of water, it undergoes a chemical reaction that produces carbon dioxide gas, resulting in the characteristic fizzing action. This mechanism is used to deliver medications and nutritional supplements in a palatable and easily dissolvable form.
- Anticoagulant in Blood Storage: Sodium citrate, a salt derived from citric acid, is widely used as an anticoagulant in blood collection and storage. It works by chelating calcium ions, which are essential for the blood clotting cascade, thereby preventing coagulation and preserving the integrity of the donated blood.
- pH Adjuster in Medications: Monohydrate citric acid is used to adjust and maintain the pH of various pharmaceutical formulations, including liquid medications, creams, and injections. Maintaining the correct pH is crucial for the stability, solubility, and bioavailability of the active pharmaceutical ingredients.
- Flavoring Agent: In some oral medications and syrups, small amounts of monohydrate citric acid may be added as a flavoring agent to mask unpleasant tastes and improve patient compliance.
- Buffering Agent: Its ability to act as a buffer system helps to maintain a stable pH in pharmaceutical solutions, preventing degradation of sensitive drugs.
D. Industrial Applications: Versatility Beyond Consumer Products
The utility of monohydrate citric acid extends beyond consumer-facing products into various industrial processes.
- Metal Chelating Agent: Its ability to chelate metal ions makes monohydrate citric acid valuable in industrial cleaning and metal finishing processes. It can be used to remove metal contaminants from surfaces and in electroplating baths to control metal ion concentration.
- Textile Industry: In the textile industry, monohydrate citric acid can be used as a mordant to prepare fabrics for dyeing and as a finishing agent to improve fabric properties.
- Water Treatment: Its chelating properties also find application in water treatment to prevent scale formation in pipes and equipment by binding to calcium and magnesium ions.
- Biodegradable Polymer Production: Citric acid is being explored as a building block for the production of biodegradable polymers, offering a sustainable alternative to traditional petroleum-based plastics.
In conclusion, the diverse applications of monohydrate citric acid underscore its remarkable versatility and importance across numerous industries. Its natural acidity, chelating properties, and ability to act as a preservative and pH adjuster make it an indispensable ingredient in countless products and processes that impact our daily lives. As environmental concerns grow, the biodegradability and relatively low toxicity of monohydrate citric acid further enhance its appeal as a sustainable and effective chemical compound.
Safety, Handling, and Storage of Monohydrate Citric Acid
While monohydrate citric acid is generally considered a safe and naturally occurring substance, particularly in the dilute concentrations found in food, it is essential to understand and adhere to proper safety protocols, handling procedures, and storage guidelines when working with it in its concentrated form. Like any chemical substance, mishandling or improper storage of monohydrate citric acid can lead to potential hazards and a degradation of its quality. This section will provide a comprehensive overview of the safety precautions, recommended handling practices, and optimal storage conditions for monohydrate citric acid to ensure both user safety and the integrity of the product.
A. Safety Precautions: Minimizing Potential Hazards
Working with concentrated monohydrate citric acid, whether in its crystalline form or in concentrated solutions, necessitates awareness of potential hazards and the implementation of appropriate safety measures.
- Irritation: Monohydrate citric acid is a mild to moderate irritant to the skin, eyes, and respiratory tract. Direct contact with the solid form or concentrated solutions can cause redness, itching, and a burning sensation. Inhalation of dust or concentrated vapors can irritate the nose, throat, and lungs, leading to coughing and shortness of breath. Therefore, it is crucial to minimize direct contact and avoid inhalation.
- Eye Contact: Eye contact with monohydrate citric acid can cause significant irritation, pain, and potentially temporary corneal damage. Immediate and thorough flushing of the eyes with copious amounts of water for at least 15-20 minutes is crucial. Medical attention should be sought promptly if irritation persists.
- Skin Contact: Prolonged or repeated skin contact with concentrated monohydrate citric acid can lead to irritation, dryness, and in some individuals, mild dermatitis. Wearing appropriate personal protective equipment (PPE), such as gloves, is essential to prevent skin contact. If contact occurs, the affected area should be washed thoroughly with soap and water.
- Ingestion: While monohydrate citric acid is commonly ingested in small amounts in food, ingestion of large quantities of the concentrated form can cause gastrointestinal irritation, including nausea, vomiting, abdominal pain, and diarrhea. Accidental ingestion should be avoided. If significant ingestion occurs, medical advice should be sought, and the individual should be monitored for any adverse effects.
- Respiratory Hazards: Handling monohydrate citric acid in its powdered form can generate dust, which can be irritating to the respiratory system upon inhalation. Operations involving powdered monohydrate citric acid should be conducted in well-ventilated areas or under a fume hood to minimize dust exposure. Respiratory protection, such as dust masks or respirators, may be necessary in situations with high dust levels.
- Reactivity: Monohydrate citric acid is generally considered stable under normal conditions. However, it can react with strong oxidizing agents, strong bases, and some metals. These reactions can potentially generate heat or flammable gases. Therefore, it should be stored away from incompatible materials.
B. Proper Handling Procedures: Best Practices for Safe Use
Implementing proper handling procedures is crucial to minimize the risks associated with working with monohydrate citric acid.
- Personal Protective Equipment (PPE): When handling monohydrate citric acid, especially in concentrated forms or powdered states, appropriate PPE should always be worn. This includes:
- Gloves: Chemical-resistant gloves (e.g., nitrile or neoprene) to prevent skin contact.
- Eye Protection: Safety glasses or goggles with side shields to protect the eyes from dust and splashes.
- Respiratory Protection: If dust or vapors are likely to be generated, a dust mask or respirator appropriate for the exposure level should be used.
- Protective Clothing: A lab coat or other protective clothing may be necessary to prevent skin contact, especially when handling large quantities.
- Engineering Controls: Utilizing engineering controls can significantly reduce exposure risks. These include:
- Ventilation: Ensuring adequate ventilation in work areas, especially when handling powdered monohydrate citric acid or preparing concentrated solutions. Local exhaust ventilation (e.g., fume hoods) should be used when necessary to capture dusts and vapors at the source.
- Containment: Using closed systems or containment measures to prevent the release of dust or solutions into the work environment.
- Safe Work Practices: Adhering to safe work practices is essential for preventing accidents and minimizing exposure:
- Avoid Generating Dust: When handling the solid form, take precautions to minimize the generation of dust.
- Handle Carefully: Avoid spilling or splashing solutions of monohydrate citric acid.
- Do Not Eat, Drink, or Smoke: Eating, drinking, or smoking should be prohibited in areas where monohydrate citric acid is handled to prevent accidental ingestion.
- Wash Hands Thoroughly: Wash hands thoroughly with soap and water after handling monohydrate citric acid, even if gloves were worn.
- Know Emergency Procedures: Be familiar with emergency procedures, including the location of eyewash stations, safety showers, and spill response equipment.
C. Optimal Storage Conditions: Maintaining Quality and Preventing Hazards
Proper storage of monohydrate citric acid is essential for maintaining its quality, preventing degradation, and minimizing potential hazards.
- Storage Area: Monohydrate citric acid should be stored in a cool, dry, and well-ventilated area. Avoid storage in areas with high humidity or extreme temperatures, as these conditions can lead to caking, degradation, or changes in its physical properties.
- Container Integrity: The product should be stored in tightly closed, original containers or other suitable, labeled containers made of compatible materials. Ensure that containers are not damaged and are properly sealed to prevent moisture absorption and contamination.
- Segregation: Monohydrate citric acid should be stored separately from incompatible materials, such as strong oxidizing agents, strong bases, and certain metals. Refer to the Safety Data Sheet (SDS) for a comprehensive list of incompatible substances.
- Labeling: All containers of monohydrate citric acid should be clearly and accurately labeled with the product name, hazard warnings, and any necessary precautions.
- Temperature and Humidity Control: Maintaining a consistent temperature and low humidity in the storage area is crucial for preventing caking and ensuring the long-term stability of monohydrate citric acid. Ideal storage temperatures are typically between 15∘C and 25∘C (59∘F and 77∘F), and relative humidity should be kept below 60%.
- Shelf Life: While monohydrate citric acid is a relatively stable compound, proper storage is essential to maximize its shelf life and maintain its quality. Refer to the manufacturer’s recommendations or the product’s Certificate of Analysis for specific shelf life information.
- Spill Response: Establish clear procedures for handling spills of monohydrate citric acid. Small spills of the solid can be swept up carefully, avoiding the generation of dust, and placed in a suitable container for disposal. Liquid spills should be contained and neutralized with a mild alkaline substance (e.g., sodium bicarbonate) before being absorbed with inert materials and disposed of according to local regulations.
By adhering to these safety precautions, handling procedures, and storage guidelines, individuals and organizations can ensure the safe and effective use of monohydrate citric acid while minimizing potential risks to human health and the environment. Always consult the Safety Data Sheet (SDS) provided by the manufacturer for detailed and specific safety information related to the particular monohydrate citric acid product being used.
Sourcing and Purchasing Monohydrate Citric Acid
For businesses and individuals seeking to utilize the versatile properties of monohydrate citric acid, understanding the landscape of sourcing and purchasing is crucial. The availability of monohydrate citric acid is widespread, but navigating the various suppliers, grades, packaging options, and quality standards requires careful consideration to ensure that the purchased product meets specific needs and regulatory requirements while optimizing cost-effectiveness. This section will provide a comprehensive guide to sourcing and purchasing monohydrate citric acid, covering key considerations for identifying reliable suppliers, understanding different product specifications, evaluating pricing and packaging, and navigating the purchasing process.
A. Identifying Suppliers: A Diverse Range of Options
The global supply chain for monohydrate citric acid is well-established, offering a variety of sourcing options to cater to different volumes and requirements. Potential buyers can typically find monohydrate citric acid through several channels:
- Chemical Distributors: These companies act as intermediaries, purchasing monohydrate citric acid in bulk from manufacturers and then reselling it in smaller quantities to a wider range of customers. Chemical distributors often offer a broad portfolio of chemical products and can provide technical support and logistical services. They can be a convenient option for businesses requiring various chemicals in moderate volumes. When selecting a chemical distributor, consider their reputation, experience in handling food-grade or industrial-grade chemicals (depending on your needs), their geographical reach, and their ability to provide the necessary documentation, such as Certificates of Analysis (COAs) and Safety Data Sheets (SDSs).
- Direct Manufacturers: Purchasing directly from manufacturers of monohydrate citric acid can be advantageous for large-volume buyers. Direct sourcing can potentially offer more competitive pricing and a more direct line of communication regarding product specifications and quality control. However, manufacturers often have minimum order quantities that may not be suitable for smaller businesses or individual users. Identifying reputable manufacturers may require market research and due diligence.
- Online Retailers and Marketplaces: The rise of e-commerce has also extended to the chemical industry, with various online retailers and marketplaces offering monohydrate citric acid in different quantities. These platforms can be convenient for smaller purchases and for comparing prices from multiple vendors. However, it is crucial to verify the reputation and reliability of online sellers, ensuring they provide adequate product information, quality documentation, and secure shipping. Exercise caution when purchasing from unfamiliar online sources and prioritize vendors with transparent product specifications and customer reviews.
- Specialty Ingredient Suppliers: Certain suppliers specialize in ingredients for specific industries, such as food, pharmaceuticals, or cosmetics. These suppliers may offer monohydrate citric acid that meets specific industry standards and certifications, along with other related ingredients and technical expertise tailored to those sectors.
B. Understanding Product Specifications and Grades: Tailoring the Purchase to Your Needs
Monohydrate citric acid is available in various grades and specifications, depending on its intended use. Understanding these distinctions is crucial for selecting the appropriate product for your application.
- Food Grade: This grade meets the stringent purity requirements for use in food and beverage products. It typically adheres to regulations set by organizations like the Food Chemicals Codex (FCC) or equivalent national and international standards. Food-grade monohydrate citric acid will have very low levels of impurities and will be manufactured under strict hygiene and quality control measures.
- Pharmaceutical Grade (USP/EP): For use in pharmaceutical and nutraceutical applications, monohydrate citric acid must meet the rigorous standards outlined in pharmacopoeias such as the United States Pharmacopeia (USP) or the European Pharmacopoeia (EP). These grades have extremely high purity levels and undergo extensive testing to ensure safety and efficacy in medicinal products.
- Industrial Grade: This grade is typically used in industrial applications such as cleaning, metal treatment, and water treatment. While it still has a certain level of purity, the requirements are generally less stringent than those for food or pharmaceutical grades. The presence of trace impurities may be acceptable as long as they do not interfere with the intended industrial process.
- Technical Grade: This is a lower purity grade suitable for some basic industrial applications where high purity is not critical. It may contain higher levels of impurities compared to other grades.
When purchasing monohydrate citric acid, always clearly specify the required grade based on your intended application. Request a Certificate of Analysis (COA) from the supplier to verify the purity and specifications of the product against the relevant standards.
C. Evaluating Pricing and Packaging: Optimizing Cost and Handling
The pricing of monohydrate citric acid can vary depending on factors such as the grade, quantity purchased, supplier, and market conditions. Packaging options also play a significant role in cost, handling, and storage.
- Pricing Factors:
- Quantity: Bulk purchases generally result in lower per-unit prices compared to smaller quantities.
- Grade: Higher purity grades, such as pharmaceutical grade, typically command a premium price due to the more stringent manufacturing and testing processes.
- Supplier: Prices can vary between different distributors and manufacturers based on their overhead costs, sourcing strategies, and profit margins.
- Market Conditions: Global supply and demand, raw material costs, and transportation expenses can influence the price of monohydrate citric acid.
- Packaging Options:Monohydrate citric acid is typically available in various packaging options, including:
- Bags: Multi-layered paper or plastic bags, often with inner liners, in sizes ranging from 25 kg to 1000 kg (FIBCs or bulk bags).
- Drums: Fiber or plastic drums of various capacities.
- Smaller Containers: For smaller quantities, it may be available in jars, bottles, or pouches.
Consider the volume of monohydrate citric acid you require, your storage capacity, and your handling equipment when selecting packaging options. Bulk bags can be cost-effective for large volumes but require specialized handling equipment. Smaller bags or drums may be more suitable for lower volumes and easier manual handling. Factor in the cost of packaging and disposal when evaluating overall purchasing costs.
D. Navigating the Purchasing Process: Key Considerations
A smooth and efficient purchasing process involves several key considerations:
- Quality Assurance: Prioritize suppliers who can provide Certificates of Analysis (COAs) for each batch of monohydrate citric acid, confirming that it meets the specified grade and purity standards. Inquire about their quality control procedures and certifications (e.g., ISO certifications).
- Regulatory Compliance: Ensure that the monohydrate citric acid you purchase complies with relevant regulations for your intended application. For food and pharmaceutical use, verify adherence to food chemical codex or pharmacopoeia standards.
- Logistics and Shipping: Consider the supplier’s shipping capabilities, lead times, and shipping costs. Ensure they can deliver the product safely and efficiently to your location. For large volumes, explore options for bulk transportation.
- Customer Support and Technical Assistance: Choose suppliers who offer good customer support and can provide technical assistance if you have questions about product specifications, handling, or applications.
- Sustainability and Ethical Sourcing: Increasingly, businesses are considering the sustainability practices and ethical sourcing of their suppliers. Inquire about the manufacturer’s environmental policies and labor practices.
- Long-Term Relationships: Building long-term relationships with reliable suppliers can often lead to better pricing, more consistent quality, and improved communication.
By carefully considering these factors throughout the sourcing and purchasing process, businesses and individuals can secure a reliable supply of high-quality monohydrate citric acid that meets their specific needs while ensuring safety, regulatory compliance, and cost-effectiveness. Thorough research, clear communication with potential suppliers, and a focus on quality assurance are essential for a successful procurement strategy.
How is Monohydrate Citric Acid Produced?
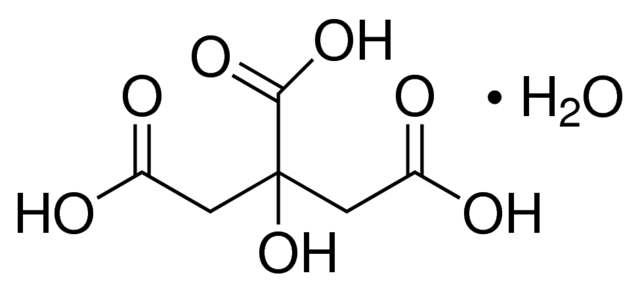
Monohydrate Citric Acid is primarily produced through fermentation. The raw materials, typically glucose or sucrose, undergo fermentation using the microorganism Aspergillus niger. This process yields citric acid, which is then purified and crystallized into its monohydrate form.
Applications of Monohydrate Citric Acid in Various Industries
Due to its multifunctional properties, Monohydrate Citric Acid is widely used across different industries. Some key applications include:
- Food & Beverage Industry: Functions as a pH regulator, natural preservative, and flavor enhancer in carbonated drinks, candies, and processed foods.
- Pharmaceuticals: Used in effervescent tablets, buffer solutions, and as an excipient in drug formulations.
- Cosmetics & Skincare: Acts as a pH adjuster and mild exfoliating agent in skincare products like toners and peeling gels.
- Chemical & Cleaning Industry: Found in household cleaning products, such as descalers for removing limescale deposits.
Safety and Regulatory Approval
The use of Monohydrate Citric Acid is approved by various regulatory agencies, including the FDA (Food and Drug Administration) in the United States and the EFSA (European Food Safety Authority) in Europe. When used within recommended limits, it is considered safe for consumption. However, excessive intake may lead to gastric irritation or dental erosion due to its acidic nature.
Differences Between Monohydrate Citric Acid and Anhydrous Citric Acid
A primary distinction between Monohydrate Citric Acid and Anhydrous Citric Acid is their water content. Monohydrate contains one water molecule per crystal unit, while anhydrous citric acid is completely devoid of water. This difference affects their stability and usage anhydrous citric acid is preferred for applications requiring moisture-free stability, whereas monohydrate citric acid is more common in liquid formulations.
| Comparison Factor | Monohydrate Citric Acid | Anhydrous Citric Acid |
| Water Content | Contains one water molecule per crystal unit | No water content |
| Physical Form | White crystals | White crystals or powder |
| Stability | More stable in liquid formulations | More stable in dry conditions |
| Common Applications | Frequently used in liquid-based products | Preferred for moisture-sensitive formulations |
| Production Process | Crystallized with water | Produced via dehydration |
Proper Storage and Handling
To maintain its quality, Monohydrate Citric Acid should be stored in a tightly sealed container in a cool, dry place. Proper storage prevents moisture absorption, which can lead to clumping and reduced effectiveness.
FAQ (Frequently Asked Questions)
Conclusion
In conclusion, monohydrate citric acid stands as a remarkably versatile and indispensable chemical compound that permeates numerous aspects of our modern world. From the tangible zest it imparts to our favorite foods and beverages to its crucial role in maintaining hygiene through effective cleaning agents and its essential functions within the pharmaceutical and industrial sectors, its impact is both widespread and profound. The unique crystalline structure, characterized by the inclusion of a single water molecule within its lattice, dictates a specific set of physical and chemical properties that underpin its diverse applications.
Throughout this exploration, we have delved into the fundamental understanding of monohydrate citric acid, dissecting its chemical formula (C6H8O7⋅H2O) and elucidating the significance of its water of hydration. We have examined its distinct physical and chemical characteristics, contrasting it with its anhydrous counterpart and highlighting the nuances that influence its behavior. Furthermore, we have traversed the extensive landscape of its applications, from its role as a flavor enhancer and preservative in the food industry to its efficacy as a descaling agent in cleaning products and its vital functions in pharmaceutical formulations and industrial processes.
Understanding the safety protocols, proper handling procedures, and optimal storage conditions for monohydrate citric acid is paramount for ensuring both user safety and the maintenance of its quality. Adhering to these guidelines minimizes potential hazards and preserves the integrity of this valuable compound. Finally, navigating the sourcing and purchasing landscape requires careful consideration of supplier reliability, product grades, packaging options, and quality assurance to secure the appropriate monohydrate citric acid for specific needs.
The enduring significance of monohydrate citric acid lies not only in its versatility but also in its relatively low toxicity and biodegradability, aligning with the growing global emphasis on sustainable practices. As research continues, we may uncover even more innovative applications for this seemingly simple yet remarkably powerful chemical. From enhancing the palatability of our meals to contributing to cleaner environments and advancing healthcare solutions, monohydrate citric acid remains a cornerstone of numerous industries, silently and effectively improving the quality and convenience of our lives. Its multifaceted nature ensures its continued importance in the years to come, solidifying its position as a truly indispensable chemical substance.

