Sodium Dihydrogen Phosphate, a versatile inorganic compound with the chemical formula NaH2PO4, stands as a significant chemical entity with a broad spectrum of applications that touch upon various aspects of our daily lives and numerous industrial processes. This white, crystalline powder, often encountered in its anhydrous or hydrated forms, plays a crucial role in fields ranging from the food we consume and the pharmaceuticals we rely on, to the treatment of our water and the very foundations of scientific research. Understanding the fundamental properties and diverse functionalities of Sodium Dihydrogen Phosphate is therefore essential for professionals and individuals alike seeking to comprehend its impact and utility in a multitude of contexts.
At its core, Sodium Dihydrogen Phosphate is a sodium salt of phosphoric acid. This means it is formed through the partial neutralization of phosphoric acid (H3PO4) with a sodium-containing base, such as sodium hydroxide (NaOH) or sodium carbonate (Na2CO3). The resulting compound retains one replaceable hydrogen atom from the phosphoric acid molecule, hence the “dihydrogen” in its name, along with the sodium cation (Na+) and the dihydrogen phosphate anion (H2PO4−). This unique chemical structure imbues Sodium Dihydrogen Phosphate with a specific set of characteristics that dictate its behavior and applicability.
One of the most notable properties of Sodium Dihydrogen Phosphate is its behavior in aqueous solutions. When dissolved in water, it exhibits a slightly acidic pH, a characteristic that underpins its use as a buffering agent. Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base, and Sodium Dihydrogen Phosphate, often in conjunction with its conjugate base, disodium hydrogen phosphate (Na2HPO4), forms an effective buffer system widely utilized in biological and chemical experiments, as well as in pharmaceutical formulations to maintain the stability and efficacy of sensitive compounds.
Beyond its buffering capabilities, Sodium Dihydrogen Phosphate finds extensive use as a food additive. In this capacity, it serves various purposes, including as an emulsifier to help mix oil and water-based ingredients, a stabilizer to prevent separation and maintain texture, and a pH adjuster to control acidity and prevent microbial growth. You can often find it listed on ingredient labels under its E number, E339(i). Its inclusion in processed foods contributes to their texture, shelf life, and overall quality.
Furthermore, the applications of Sodium Dihydrogen Phosphate extend into the realm of water treatment. It acts as a corrosion inhibitor in water systems, preventing the degradation of metal pipes and equipment. Its ability to control pH also makes it valuable in optimizing various water treatment processes. In the pharmaceutical industry, besides its role as a buffer, it can also function as an excipient, an inactive substance that serves as the vehicle or medium for a drug.
In the scientific laboratory, Sodium Dihydrogen Phosphate is an indispensable reagent. Its well-defined properties and consistent behavior make it a crucial component in countless experimental protocols, analytical techniques, and the preparation of various solutions. From biological research to chemical synthesis, its reliability ensures the accuracy and reproducibility of scientific findings.
This article aims to delve deeper into the multifaceted nature of Sodium Dihydrogen Phosphate. We will explore its fundamental chemical and physical properties, unravel the diverse array of its applications across various industries, and discuss the essential safety considerations associated with its handling and use. By providing a comprehensive overview, we intend to illuminate the significance of this seemingly simple chemical compound and its profound impact on our modern world.
- Chemical and Physical Properties
- Uses and Applications
- Safety and Handling
- Synthesis and Production
- Properties of Sodium Dihydrogen Phosphate
- Uses of Sodium Dihydrogen Phosphate
- Safety of Sodium Dihydrogen Phosphate
- Role of Sodium Dihydrogen Phosphate in Detergents
- Solubility of Sodium Dihydrogen Phosphate
- Conclusion
- FAQ (Frequently Asked Questions)
Chemical and Physical Properties
Understanding the chemical and physical properties of Sodium Dihydrogen Phosphate is fundamental to appreciating its diverse applications and handling requirements. This section will delve into the molecular structure, molar mass, physical appearance, solubility characteristics, acidic nature, buffering capacity, and other pertinent physical attributes of this important compound.
A. Chemical Structure and Formula
At the molecular level, Sodium Dihydrogen Phosphate is an ionic compound composed of a positively charged sodium cation (Na+) and a negatively charged dihydrogen phosphate anion (H2PO4−). The chemical formula, NaH2PO4, accurately reflects this 1:1 stoichiometric ratio. The dihydrogen phosphate anion is derived from phosphoric acid (H3PO4), a weak inorganic acid containing a central phosphorus atom bonded to four oxygen atoms. One of these oxygen atoms is double-bonded to the phosphorus, while the other three are single-bonded, each carrying a hydrogen atom. In the formation of the dihydrogen phosphate ion, two of these hydrogen atoms are lost, resulting in a net negative charge on the ion. This negative charge is then balanced by the positive charge of the sodium ion, forming the neutral compound Sodium Dihydrogen Phosphate.
The structure can be visualized with the phosphorus atom at the center, tetrahedrally surrounded by four oxygen atoms. Two of these oxygen atoms are bonded to hydrogen atoms (hence “dihydrogen”), one is double-bonded to the phosphorus, and the remaining oxygen atom carries the negative charge that attracts the sodium ion. This ionic bond between the sodium cation and the dihydrogen phosphate anion is responsible for many of the physical properties observed in Sodium Dihydrogen Phosphate.
B. Molar Mass
The molar mass of Sodium Dihydrogen Phosphate is a crucial property for stoichiometric calculations in chemical reactions and for preparing solutions of specific concentrations. To calculate the molar mass, we sum the atomic masses of all the atoms present in the chemical formula (NaH2PO4). Using the standard atomic masses:
- Sodium (Na): approximately 22.99 g/mol
- Hydrogen (H): approximately 1.01 g/mol
- Phosphorus (P): approximately 30.97 g/mol
- Oxygen (O): approximately 16.00 g/mol
Therefore, the molar mass of anhydrous Sodium Dihydrogen Phosphate is:
M(NaH2PO4)=1×M(Na)+2×M(H)+1×M(P)+4×M(O) M(NaH2PO4)=(1×22.99)+(2×1.01)+(1×30.97)+(4×16.00) M(NaH2PO4)=22.99+2.02+30.97+64.00 M(NaH2PO4)=120.0g/mol
It’s important to note that Sodium Dihydrogen Phosphate can also exist in hydrated forms, most commonly as the monohydrate (NaH2PO4⋅H2O) and the dihydrate (NaH2PO4⋅2H2O). The molar mass of these hydrated forms would include the mass of the water molecules associated with each formula unit. For the monohydrate, the molar mass would be 120.0g/mol+18.02g/mol(H2O)=138.02g/mol.
C. Physical Appearance
At room temperature, anhydrous Sodium Dihydrogen Phosphate typically presents as a white, odorless, crystalline powder. The crystalline structure arises from the ordered arrangement of the sodium and dihydrogen phosphate ions in a lattice. The hydrated forms also appear as white crystalline solids, although their crystal structure may differ slightly due to the inclusion of water molecules within the lattice. The appearance can sometimes vary depending on the particle size and the presence of any impurities, but generally, it is a free-flowing, white solid.
D. Solubility
Sodium Dihydrogen Phosphate exhibits good solubility in water, which is a crucial property for its applications in aqueous solutions, such as buffers and food additives. The polar nature of water molecules allows them to effectively solvate the charged sodium and dihydrogen phosphate ions, disrupting the ionic lattice and leading to dissolution. The solubility of Sodium Dihydrogen Phosphate in water is temperature-dependent, generally increasing with temperature, although the extent of this increase varies.
Its solubility in other solvents is considerably lower. It is generally insoluble or poorly soluble in nonpolar organic solvents such as hexane, benzene, and diethyl ether. Solubility in polar organic solvents like ethanol and methanol is limited and depends on the specific solvent and temperature. This differential solubility is often exploited in separation and purification processes.
E. pH and Buffering Capacity
When Sodium Dihydrogen Phosphate dissolves in water, it forms a slightly acidic solution. This is due to the amphoteric nature of the dihydrogen phosphate ion (H2PO4−), which can act as both an acid and a base. In water, it undergoes the following equilibrium reactions:
H2PO4−(aq)+H2O(l)⇌HPO42−(aq)+H3O+(aq)(Ka2≈6.2×10−8) H2PO4−(aq)+H2O(l)⇌H3PO4(aq)+OH−(aq)(Kb3=Ka1Kw≈1.3×10−12)
The first equilibrium, with a Ka2 value of approximately 6.2×10−8, indicates a weak acidic behavior, while the second equilibrium, with a very small Kb3 value, indicates a very weak basic behavior. Since Ka2>Kb3, aqueous solutions of Sodium Dihydrogen Phosphate are slightly acidic, typically with a pH in the range of 4 to 5, depending on the concentration.
The real power of Sodium Dihydrogen Phosphate in solution arises when it is used in conjunction with its conjugate base, disodium hydrogen phosphate (Na2HPO4). This mixture forms a highly effective buffer system that can resist significant changes in pH upon the addition of moderate amounts of acid or base. The buffering action is based on the equilibrium between the weak acid (H2PO4−) and its conjugate base (HPO42−):
H2PO4−(aq)⇌H+(aq)+HPO42−(aq)
If an acid (H+) is added to the buffer solution, it reacts with the HPO42− ions to form H2PO4−, minimizing the change in pH. Conversely, if a base (OH−) is added, it reacts with the H2PO4− ions to form HPO42− and water, again resisting a drastic pH increase. The optimal buffering capacity of this system is around its pKa value, which is approximately 7.2. This makes Sodium Dihydrogen Phosphate and its disodium salt an indispensable buffer system in biological and biochemical applications where maintaining a specific pH is critical.
F. Other Key Properties
Beyond the properties already discussed, Sodium Dihydrogen Phosphate exhibits other notable physical characteristics. Its melting point is relatively high, although it often decomposes upon heating before reaching a distinct melting point, losing water of hydration if present and eventually forming sodium metaphosphate (NaPO3) at higher temperatures.
The density of anhydrous Sodium Dihydrogen Phosphate is approximately 2.04 g/cm³. The hydrated forms have slightly different densities.
Sodium Dihydrogen Phosphate is generally non-hygroscopic in its anhydrous form under normal conditions, meaning it does not readily absorb moisture from the air. However, the hydrated forms can lose water upon heating or under low humidity conditions.
In summary, Sodium Dihydrogen Phosphate is a white crystalline solid with good water solubility, forming slightly acidic solutions. Its ability to act as a buffer, especially in combination with its conjugate base, is a key chemical property that underpins many of its applications. Understanding these chemical and physical properties is crucial for its effective and safe utilization across various scientific, industrial, and everyday applications.
Uses and Applications
The unique chemical and physical properties of Sodium Dihydrogen Phosphate underpin its remarkably diverse range of uses and applications across numerous industries and scientific disciplines. From the food we consume to the intricate processes of pharmaceutical manufacturing and the fundamental research conducted in laboratories, this compound plays a vital role. Its versatility stems primarily from its ability to act as a buffering agent, its properties as a food additive, its role in water treatment, and its utility as a laboratory reagent. This section will explore these key areas and delve into the specific ways Sodium Dihydrogen Phosphate is utilized.
A. Food Industry
In the food industry, Sodium Dihydrogen Phosphate serves as a multifaceted ingredient, contributing to the texture, stability, and safety of a wide array of food products. It is often employed as an emulsifier, aiding in the dispersion of fats and oils in water-based systems, preventing separation and ensuring a homogenous mixture. This is particularly important in processed foods like cheese spreads, where it helps to create a smooth and consistent texture.
As a stabilizer, Sodium Dihydrogen Phosphate helps to maintain the desired physical and chemical characteristics of food products over time. It can prevent the precipitation of proteins in processed meats, improve the texture of canned goods, and enhance the stability of dairy products. By interacting with various food components, it contributes to the overall quality and shelf life of these items.
Furthermore, Sodium Dihydrogen Phosphate acts as a pH adjuster. Controlling the acidity or alkalinity of food is crucial for various reasons, including influencing flavor, texture, and preventing the growth of spoilage microorganisms. In baked goods, it can interact with leavening agents to control the rate of gas production. In processed cheeses, it helps to achieve the desired pH for melting and texture. The ability of Sodium Dihydrogen Phosphate to contribute to a specific pH range makes it an invaluable tool in food formulation.
Specific examples of food products where Sodium Dihydrogen Phosphate (often listed under its E number, E339(i)) can be found include:
- Processed Cheeses: As an emulsifying salt to create a smooth, meltable product.
- Baked Goods: As a component of leavening systems and to adjust pH.
- Processed Meats: To improve water retention and texture.
- Canned Goods: To stabilize the product and prevent discoloration.
- Beverages: As a buffering agent to maintain pH.
- Dairy Products: To stabilize proteins and control viscosity.
The use of Sodium Dihydrogen Phosphate in the food industry is carefully regulated to ensure safety and efficacy, with permissible limits defined by food safety authorities worldwide.
B. Pharmaceutical Industry
The pharmaceutical industry leverages the buffering capacity and other properties of Sodium Dihydrogen Phosphate in various applications critical to drug formulation and delivery. Its ability to maintain a stable pH is paramount in ensuring the efficacy and stability of many pharmaceutical preparations.
As a buffering agent, Sodium Dihydrogen Phosphate, often in combination with its conjugate base, disodium hydrogen phosphate, is used to create buffer solutions for injectable drugs, ophthalmic solutions, and oral medications. Maintaining the correct pH is essential for the solubility, stability, and physiological compatibility of these formulations. For instance, injectable drugs need to be formulated at a pH that is compatible with blood to avoid adverse reactions upon administration.
Sodium Dihydrogen Phosphate can also act as an excipient, an inactive ingredient that serves as a carrier or vehicle for the active pharmaceutical ingredient. It can aid in the dissolution or dispersion of the drug, improve its bioavailability, or contribute to the overall physical characteristics of the dosage form, such as tablets or solutions.
Furthermore, it can be used in the preparation of phosphate buffers for various biological and biochemical assays conducted in pharmaceutical research and quality control laboratories. These buffers provide a stable environment for studying the behavior of drugs and biological molecules.
C. Water Treatment
In water treatment, Sodium Dihydrogen Phosphate plays a significant role in preventing corrosion and controlling pH in water systems. As a corrosion inhibitor, it can form a protective layer on the surface of metal pipes and equipment, preventing the dissolution of metal ions into the water and thus reducing the risk of pipe damage and the contamination of water with heavy metals. This is particularly important in industrial water systems, cooling towers, and municipal water supplies.
Its ability to adjust pH is also crucial in optimizing various water treatment processes, such as coagulation and disinfection. Maintaining the correct pH range ensures the effectiveness of these processes and the overall quality of the treated water.
D. Laboratory and Research
The consistent and well-defined properties of Sodium Dihydrogen Phosphate make it an indispensable reagent in various laboratory and research settings. Its primary use is in the preparation of buffer solutions for a wide range of biological, biochemical, and chemical experiments. Phosphate buffers, made from mixtures of Sodium Dihydrogen Phosphate and disodium hydrogen phosphate, are widely used due to their effectiveness in the physiological pH range (around 7.2) and their compatibility with many biological systems.
These buffers are essential for:
- Maintaining the pH of cell culture media: Ensuring optimal conditions for cell growth and function.
- Enzyme assays: Providing a stable pH environment for enzymatic reactions.
- DNA and protein purification: Maintaining the integrity and solubility of biomolecules during separation and analysis.
- Chromatography: As a component of mobile phases to control pH and ionic strength.
- Electrophoresis: To provide a stable pH for the separation of molecules based on charge and size.
Beyond its use in buffers, Sodium Dihydrogen Phosphate can also serve as a source of phosphate ions in various chemical reactions and analytical techniques. It is used in the preparation of other phosphate salts and as a reagent in specific chemical analyses.
E. Agriculture
In agriculture, Sodium Dihydrogen Phosphate can be a component of certain fertilizers, providing a source of phosphorus, an essential macronutrient for plant growth and development. Phosphorus plays a crucial role in energy transfer, root development, and flowering. While other phosphate fertilizers are more commonly used due to their higher phosphorus content, Sodium Dihydrogen Phosphate may be included in specific formulations depending on the soil conditions and the needs of the crop.
F. Other Industrial Applications
While the applications mentioned above are the most prominent, Sodium Dihydrogen Phosphate finds use in other specialized industrial processes as well. For instance, it can be used in certain ceramic and glass manufacturing processes. It may also have niche applications in metal finishing and textile processing.
In conclusion, the versatility of Sodium Dihydrogen Phosphate is a testament to its fundamental chemical properties. Its ability to buffer solutions, modify food texture and stability, inhibit corrosion in water systems, and serve as a crucial reagent in scientific research makes it an indispensable compound across a wide spectrum of human endeavors. As our understanding of its properties continues to evolve, new and innovative applications for Sodium Dihydrogen Phosphate are likely to emerge.
Safety and Handling
While Sodium Dihydrogen Phosphate is generally considered a relatively safe compound with numerous beneficial applications, it is crucial to understand and adhere to proper safety and handling procedures to minimize potential risks to human health and the environment. This section will detail the potential hazards associated with Sodium Dihydrogen Phosphate, outline necessary first aid measures in case of exposure, and provide comprehensive guidelines for safe handling, storage, and disposal.
A. Potential Hazards
Exposure to Sodium Dihydrogen Phosphate can pose certain health hazards, primarily through inhalation, ingestion, skin contact, and eye contact. The severity of these effects can depend on the concentration of the substance, the duration of exposure, and the individual’s susceptibility.
- Skin Contact: Direct contact with Sodium Dihydrogen Phosphate can cause mild to moderate skin irritation in some individuals. This may manifest as redness, itching, and dryness. Prolonged or repeated exposure may lead to dermatitis, an inflammation of the skin characterized by redness, swelling, and blistering. While it is not considered a strong skin irritant, precautions should be taken to avoid prolonged contact.
- Eye Contact: Contact with Sodium Dihydrogen Phosphate can cause eye irritation, ranging from mild discomfort and redness to more severe stinging, tearing, and potential corneal abrasion if the exposure is significant and not promptly addressed. The finely powdered form poses a greater risk of causing mechanical irritation to the eyes.
- Inhalation: Inhalation of Sodium Dihydrogen Phosphate dust or fine particles can irritate the respiratory tract, including the nose, throat, and lungs. This may lead to coughing, sneezing, and shortness of breath, particularly in individuals with pre-existing respiratory conditions such as asthma or bronchitis. Prolonged or excessive inhalation of dust may cause more significant respiratory issues.
- Ingestion: Ingestion of small amounts of Sodium Dihydrogen Phosphate is not typically associated with severe systemic toxicity. However, large doses may cause gastrointestinal irritation, including nausea, vomiting, abdominal pain, and diarrhea. The acidic nature of the compound in solution can contribute to this irritation.
- Chronic Effects: Limited information is available regarding the long-term chronic effects of exposure to Sodium Dihydrogen Phosphate in humans. However, based on its chemical properties, prolonged and repeated exposure to dust may potentially lead to chronic respiratory issues. Prudent handling practices aim to minimize any potential for chronic effects.
- Environmental Hazards: Sodium Dihydrogen Phosphate is generally not considered to be a significant environmental hazard in typical usage scenarios. It is a source of phosphate, which is a nutrient for plants and algae. However, excessive release into aquatic environments can contribute to eutrophication, an over-enrichment of water bodies with nutrients, leading to algal blooms and oxygen depletion. Therefore, proper disposal is essential to prevent environmental contamination.
B. First Aid Measures
In the event of exposure to Sodium Dihydrogen Phosphate, immediate and appropriate first aid measures should be taken:
- Skin Contact: Immediately flush the affected area with copious amounts of water for at least 15 minutes. Remove any contaminated clothing and shoes. If irritation persists, seek medical attention. Wash contaminated clothing before reuse.
- Eye Contact: Immediately flush the eyes with a gentle stream of water for at least 15 minutes, holding the eyelids open to ensure thorough rinsing. If irritation persists, seek immediate medical attention, preferably from an ophthalmologist.
- Inhalation: Move the affected person to fresh air immediately. If breathing is difficult, administer oxygen. If the person is not breathing, administer artificial respiration. Seek immediate medical attention.
- Ingestion: If the person is conscious, have them drink plenty of water to dilute the substance in the stomach. Do not induce vomiting unless directed to do so by medical personnel. Seek medical attention or contact a poison control center immediately.
- General Advice: Always have the Safety Data Sheet (SDS) for Sodium Dihydrogen Phosphate readily available and consult it for more specific information. Ensure that medical personnel are aware of the specific substance involved in any exposure incident.
C. Handling Precautions
To ensure the safe handling of Sodium Dihydrogen Phosphate, the following precautions should be implemented:
- Personal Protective Equipment (PPE): Wear appropriate PPE, including safety glasses with side shields, chemical-resistant gloves (e.g., nitrile or neoprene), and a lab coat or apron to prevent skin and eye contact. If there is a risk of dust generation or inhalation, wear a dust mask or respirator approved for particulate matter.
- Engineering Controls: Use adequate ventilation, such as a fume hood or local exhaust ventilation, when handling Sodium Dihydrogen Phosphate, especially in powdered form, to minimize the risk of inhalation.
- Safe Handling Practices: Avoid generating dust. Handle the substance in a well-ventilated area. Avoid contact with skin, eyes, and clothing. Do not ingest or inhale the substance. Wash hands thoroughly with soap and water after handling and before eating, drinking, or smoking.
- Spills and Leaks: In case of a spill, contain the spill immediately and clean it up using appropriate methods. Avoid dry sweeping, which can generate dust. Use a wet sweeping method or a vacuum cleaner equipped with a HEPA filter. Wear appropriate PPE during cleanup. Dispose of the spilled material according to local regulations.
- Hygiene Measures: Practice good personal hygiene. Remove contaminated clothing promptly and wash it separately. Do not eat, drink, or smoke in areas where Sodium Dihydrogen Phosphate is handled or stored.
D. Storage Conditions
Proper storage is essential to maintain the quality and safety of Sodium Dihydrogen Phosphate:
- Storage Area: Store in a cool, dry, and well-ventilated area away from incompatible materials such as strong acids and strong bases.
- Container Integrity: Keep containers tightly closed to prevent moisture absorption and contamination. Use appropriate containers made of compatible materials.
- Temperature and Humidity: Store at room temperature and avoid excessive humidity, especially for hydrated forms, which may lose or gain water depending on the conditions.
- Labeling: Ensure that containers are clearly labeled with the name of the substance, hazard warnings, and handling instructions.
- Segregation: Store away from foodstuff and beverages to prevent accidental contamination.
E. Disposal Considerations
Disposal of Sodium Dihydrogen Phosphate should be carried out in accordance with local, regional, and national environmental regulations.
- Waste Disposal: Do not dispose of Sodium Dihydrogen Phosphate down the drain or in general waste. Consult local environmental authorities or a licensed waste disposal contractor for proper disposal methods.
- Container Disposal: Dispose of empty containers in accordance with local regulations. Ensure that containers are properly cleaned before disposal or recycling.
By adhering to these safety and handling guidelines, the risks associated with the use of Sodium Dihydrogen Phosphate can be significantly minimized, ensuring a safe working environment and responsible environmental practices. Always consult the Safety Data Sheet (SDS) for the most up-to-date and comprehensive information regarding the hazards, handling, storage, and disposal of Sodium Dihydrogen Phosphate.
Synthesis and Production
The synthesis and production of Sodium Dihydrogen Phosphate are well-established industrial processes that rely on fundamental chemical reactions involving phosphoric acid and a sodium-containing base. The scale of production ranges from laboratory synthesis for research purposes to large-scale industrial manufacturing to meet the demands of various applications, including food, pharmaceuticals, and water treatment. Different production methods exist, each with its own set of reaction conditions, efficiency, and resulting product characteristics. This section will explore the primary methods employed for the synthesis and production of Sodium Dihydrogen Phosphate, along with the underlying chemical principles and process considerations.
The most common route for the production of Sodium Dihydrogen Phosphate involves the controlled neutralization of phosphoric acid (H3PO4) with a sodium-containing compound, typically sodium carbonate (Na2CO3) or sodium hydroxide (NaOH). The stoichiometry of the reaction is carefully controlled to ensure the formation of the dihydrogen phosphate salt (NaH2PO4) as the predominant product.
A. Reaction with Sodium Carbonate
One widely used method involves the reaction between phosphoric acid and sodium carbonate. Sodium carbonate is a relatively inexpensive and readily available alkali. The reaction proceeds in a stepwise manner, with the initial formation of sodium bicarbonate (NaHCO3), which then further reacts with phosphoric acid to yield Sodium Dihydrogen Phosphate, water, and carbon dioxide gas. The overall reaction can be represented as:
Na2CO3(s)+2H3PO4(aq)→2NaH2PO4(aq)+H2O(l)+CO2(g)
In practice, the process involves the slow addition of solid sodium carbonate to a dilute aqueous solution of phosphoric acid under controlled stirring. The rate of addition is carefully managed to prevent excessive foaming due to the evolution of carbon dioxide gas. The reaction is exothermic, so temperature control may be necessary to maintain optimal reaction conditions and prevent side reactions.
The resulting solution of Sodium Dihydrogen Phosphate is then typically subjected to further processing to obtain the solid product. This usually involves evaporation of the water to concentrate the solution, followed by crystallization. The crystallization process can be influenced by factors such as temperature, concentration, and the presence of seed crystals. After crystallization, the solid Sodium Dihydrogen Phosphate is separated from the mother liquor by filtration or centrifugation, washed to remove any residual impurities, and then dried to obtain the final product in the desired anhydrous or hydrated form. The specific hydration state (anhydrous, monohydrate, or dihydrate) can be controlled by adjusting the crystallization conditions and the drying temperature.
B. Reaction with Sodium Hydroxide
Another common method for producing Sodium Dihydrogen Phosphate involves the reaction between phosphoric acid and sodium hydroxide. Sodium hydroxide is a strong base that reacts readily and exothermically with phosphoric acid. The reaction must be carefully controlled to ensure the formation of the monobasic salt. The overall reaction is:
NaOH(aq)+H3PO4(aq)→NaH2PO4(aq)+H2O(l)
In industrial settings, a concentrated solution of sodium hydroxide is slowly added to a dilute solution of phosphoric acid with continuous mixing and temperature monitoring. The heat generated by the reaction must be effectively dissipated to prevent boiling and potential hazards. The reaction is typically carried out at a controlled pH to ensure the desired product is formed with minimal formation of dibasic (Na2HPO4) or tribasic (Na3PO4) sodium phosphates.
Similar to the process using sodium carbonate, the resulting solution of Sodium Dihydrogen Phosphate is then concentrated by evaporation and crystallized. The crystals are separated, washed, and dried to yield the final product. The control of crystallization conditions allows for the production of Sodium Dihydrogen Phosphate with specific particle sizes and hydration states, tailored to the requirements of different applications.
C. Laboratory Synthesis
In a laboratory setting, the synthesis of Sodium Dihydrogen Phosphate often follows similar principles but on a smaller scale. For instance, a researcher might carefully titrate a known concentration of phosphoric acid with a standardized solution of sodium hydroxide or sodium carbonate until the desired pH for the formation of Sodium Dihydrogen Phosphate is reached. The solution can then be evaporated to obtain the solid salt. The focus in laboratory synthesis is often on achieving high purity and characterizing the product, rather than large-scale production efficiency.
D. Production of Different Hydrates
Sodium Dihydrogen Phosphate can exist in anhydrous form (NaH2PO4) as well as in hydrated forms, most commonly the monohydrate (NaH2PO4⋅H2O) and the dihydrate (NaH2PO4⋅2H2O). The specific hydrate formed during the crystallization process is largely influenced by the temperature and concentration of the solution.
- Anhydrous Sodium Dihydrogen Phosphate: Crystallization from a concentrated solution at higher temperatures generally favors the formation of the anhydrous form. Drying the hydrated forms at sufficiently high temperatures can also yield the anhydrous salt, but careful control is needed to avoid decomposition.
- Sodium Dihydrogen Phosphate Monohydrate: Crystallization at moderate temperatures from a solution with a specific water concentration often leads to the formation of the monohydrate.
- Sodium Dihydrogen Phosphate Dihydrate: Crystallization from cooler solutions with a higher water content typically results in the formation of the dihydrate.
Industrial production processes are often designed to yield the specific hydrate required for the intended application, optimizing the crystallization and drying steps accordingly.
E. Quality Control and Purification
Throughout the synthesis and production process, rigorous quality control measures are implemented to ensure the purity and consistency of the final Sodium Dihydrogen Phosphate product. These measures may include monitoring the pH of the reaction mixture, controlling the temperature and concentration during crystallization, and analyzing the final product for assay (purity), heavy metal content, and other relevant specifications.
Purification steps may be necessary to remove any impurities introduced during the raw material sourcing or the production process. Recrystallization from water is a common purification technique used to obtain Sodium Dihydrogen Phosphate of higher purity. The process involves dissolving the crude product in water at an elevated temperature, followed by slow cooling to allow for the formation of purer crystals, which are then separated and dried.
F. Raw Materials and Economic Considerations
The primary raw materials for the production of Sodium Dihydrogen Phosphate are phosphoric acid and a sodium-containing base (sodium carbonate or sodium hydroxide). The quality and cost of these raw materials significantly impact the overall economics of the production process. Phosphoric acid is typically produced from phosphate rock, while sodium carbonate is often obtained from natural deposits or synthesized through the Solvay process, and sodium hydroxide is primarily produced through the electrolysis of brine.
The choice between using sodium carbonate and sodium hydroxide often depends on factors such as the cost and availability of the raw materials, the desired purity of the final product, and the specific process design. The byproduct carbon dioxide generated in the reaction with sodium carbonate may have some economic value or require disposal. The highly exothermic nature of the reaction with sodium hydroxide necessitates efficient heat management.
In conclusion, the synthesis and production of Sodium Dihydrogen Phosphate are based on well-understood chemical neutralization reactions. Industrial processes are optimized to produce large quantities of the product with controlled purity and physical properties, including hydration state and particle size, to meet the diverse needs of its various applications. Careful control of reaction conditions, crystallization, and purification steps are crucial for ensuring the quality and efficiency of the production of this important chemical compound.
Properties of Sodium Dihydrogen Phosphate
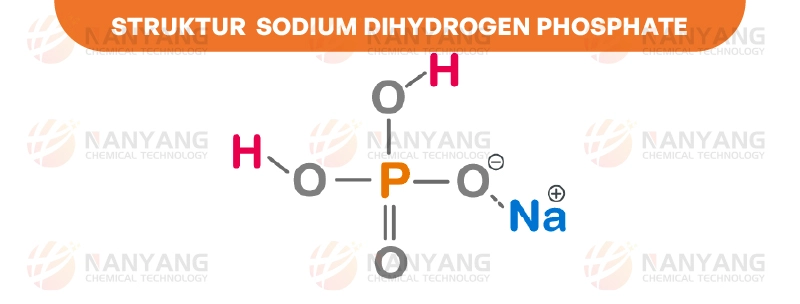
Sodium Dihydrogen Phosphate has several key physical and chemical properties that make it valuable in a range of applications. The compound is highly soluble in water and forms a mildly acidic solution.
One of its most important properties is its buffering capability, which allows it to maintain stable pH levels in different chemical and biological processes. This property is especially useful in laboratory experiments and industrial processes.
Uses of Sodium Dihydrogen Phosphate
Sodium Dihydrogen Phosphate has various applications across different sectors. In water treatment, it is used to control pH and prevent corrosion in water systems. The compound is also commonly used in the food industry to regulate pH levels in beverages, dairy products, and other foods.
Additionally, Sodium Dihydrogen Phosphate is used in detergents, where it improves the effectiveness of cleaning agents.
1. Sodium Dihydrogen Phosphate in Water Treatment
Sodium Dihydrogen Phosphate plays a key role in water treatment processes, where it is used to adjust pH and maintain the proper acidity levels in water.
Its buffering properties help prevent corrosion and scale formation, improving the efficiency of water treatment systems.
2. Sodium Dihydrogen Phosphate in the Food Industry
In the food industry, Sodium Dihydrogen Phosphate is utilized to control the pH of various food and beverage products.
Its ability to stabilize the pH helps maintain the quality, taste, and shelf life of products such as cheese, soft drinks, and processed foods.
3. Sodium Dihydrogen Phosphate in Industry and Laboratories
Sodium Dihydrogen Phosphate is essential in laboratory settings, particularly as a chemical reagent in various reactions. It is also used to prepare buffer solutions for maintaining pH stability in biochemical and chemical experiments.
As a buffer, it helps preserve the pH balance necessary for many enzymatic reactions and biological processes.
a. Chemical Reagents for Laboratory
In laboratories, Sodium Dihydrogen Phosphate is frequently used to create solutions with precise pH values. This is crucial for conducting experiments where maintaining a consistent pH is vital to ensure reproducible results.
b. Buffer Solutions for pH Adjustment
One of the primary uses of Sodium Dihydrogen Phosphate in laboratories is to prepare buffer solutions that regulate pH. By preventing drastic pH changes, these buffers are indispensable in chemical and biological studies where pH stability is critical.
Safety of Sodium Dihydrogen Phosphate
Like any chemical compound, Sodium Dihydrogen Phosphate must be handled with care. While it is not highly hazardous, precautions should be taken to avoid skin and eye irritation.
Wearing protective gloves and goggles is recommended when working with this substance. Additionally, it should be stored in a dry, cool place to maintain its stability.
Also read: Effective of Poly Aluminum Chloride (PAC) for Water Treatment
Role of Sodium Dihydrogen Phosphate in Detergents
Sodium Dihydrogen Phosphate plays an important role in detergents and cleaning products. It enhances the effectiveness of cleaning agents by stabilizing the pH and reducing the hardness of water. This allows detergents to work more efficiently, particularly in removing stains and dirt from various surfaces.
Solubility of Sodium Dihydrogen Phosphate
Sodium Dihydrogen Phosphate is highly soluble in water, which allows it to be easily incorporated into solutions for various applications. Its solubility is particularly important in water treatment and laboratory experiments, where accurate concentrations of the compound are necessary for effective pH control.
Conclusion
In summary, Sodium Dihydrogen Phosphate stands as a remarkably versatile chemical compound with a significant impact across a wide array of sectors. Its unique chemical structure and resulting properties, particularly its buffering capacity and behavior as a food additive, underpin its diverse applications. From ensuring the stable pH of vital pharmaceutical formulations and biological research experiments to contributing to the texture and preservation of our food supply, Sodium Dihydrogen Phosphate plays a crucial, often незаметную, role in modern life. Its effectiveness as a corrosion inhibitor in water treatment further highlights its utility in maintaining infrastructure and public health.
Understanding the fundamental chemical and physical properties of Sodium Dihydrogen Phosphate is essential for its safe and effective utilization. Proper handling procedures, adherence to safety guidelines, and responsible disposal methods are paramount to minimize any potential risks associated with its use. The well-established synthesis and production processes, often involving the controlled neutralization of phosphoric acid with sodium-containing bases, allow for the large-scale availability of this important compound in various forms, including anhydrous and hydrated states, tailored to specific application requirements.
The continued research and development in fields such as food science, pharmaceuticals, and materials science may uncover even more novel applications for Sodium Dihydrogen Phosphate in the future. Its relatively low toxicity and established safety profile in regulated applications further contribute to its widespread use. As we continue to seek innovative solutions in various industries, Sodium Dihydrogen Phosphate will likely remain a key chemical component, demonstrating the importance of seemingly simple inorganic compounds in addressing complex challenges and enhancing the quality of life. Its multifaceted nature underscores the interconnectedness of chemistry with numerous aspects of our technological and societal advancements.

